Label: FEXOFENADINE HYDROCHLORIDE tablet
- NDC Code(s): 55111-782-01, 55111-782-30, 55111-782-78, 55111-782-79, view more
- Packager: Dr. Reddy's Laboratories Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient(s)Fexofenadine HCl USP, 30 mg - Fexofenadine HCl USP, 60 mg - Fexofenadine HCl USP, 180 mg
-
PurposeAntihistamine
-
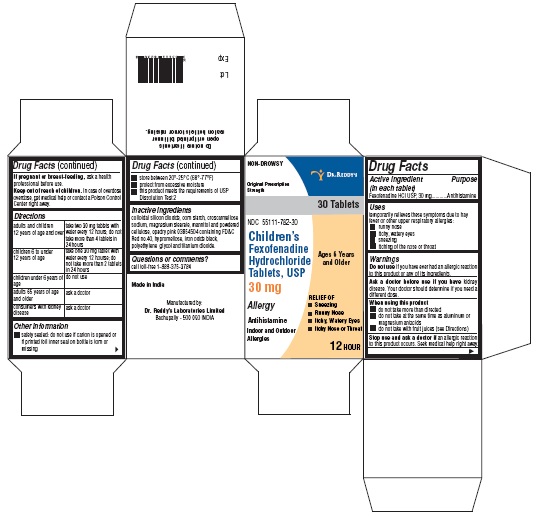
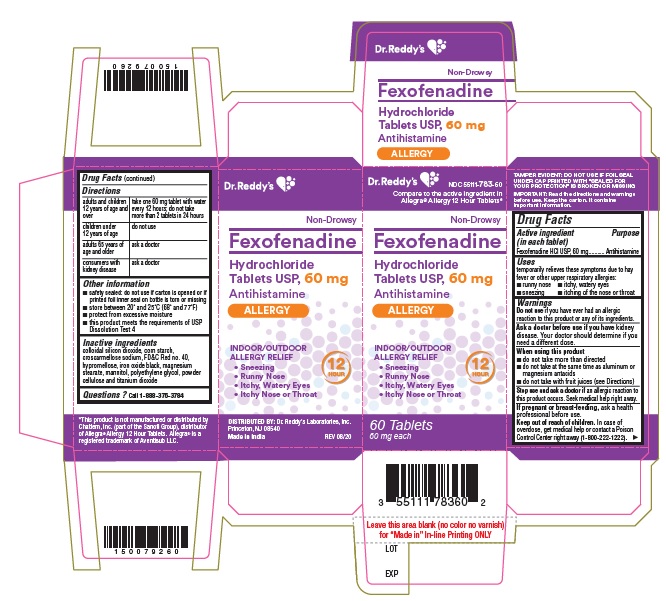
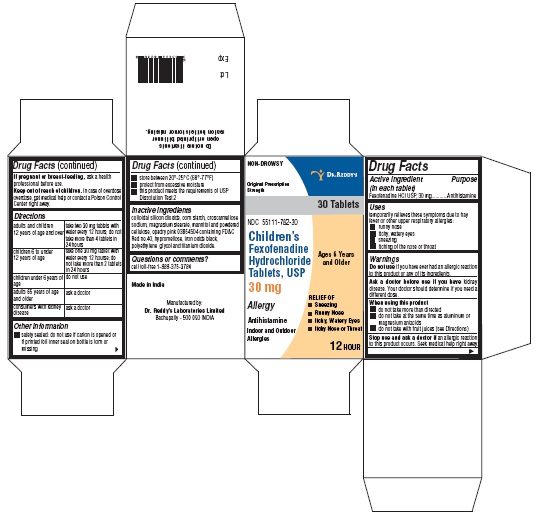
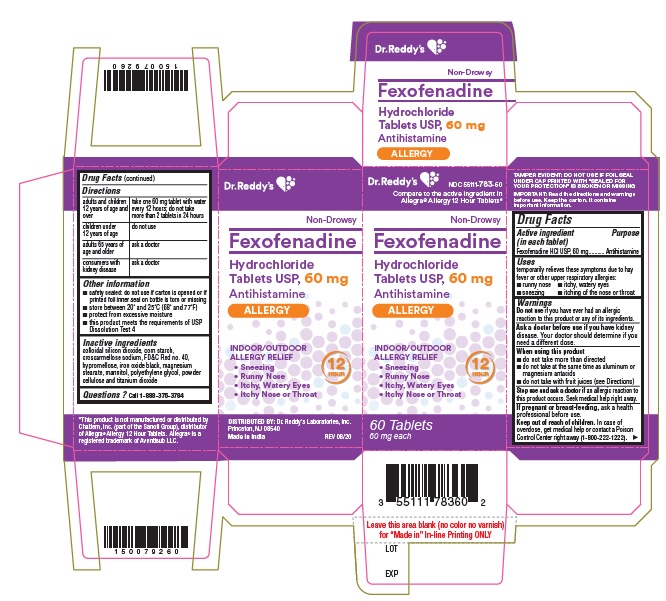
Use(s)temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose - itchy, watery eyes - sneezing - itching of the nose or throat
-
WarningsDo not use - if you have ever had an allergic reaction to this product or any of its ingredients. Ask a doctor before use if you have - kidney disease. Your doctor should determine if you ...
-
Directionsadults and children 12 years of age and over take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours - children under 12 years of agedo not use - adults ...
-
Other informationsafety sealed: do not use if carton is opened or if printed foil inner seal on bottle is torn or missing - store between 20° and 25°C (68° and 77°F) protect from excessive moisture - this product ...
-
Inactive ingredientscolloidal silicon dioxide, corn starch, croscarmellose sodium, FD&C Red no. 40, hypromellose, iron oxide black, magnesium stearate, mannitol, polyethylene glycol, powder cellulose and titanium ...
-
Questions?Call 1-888-375-3784
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL SECTIONBottle label:
-
PRINCIPAL DISPLAY PANELFexofenadine HCl Tablets, 30 mg Carton:
-
PRINCIPAL DISPLAY PANELFexofenadine HCl Tablets USP, 60 mg Carton:
-
PRINCIPAL DISPLAY PANELFexofenadine HCl Tablets USP, 180 mg Carton Label:
-
INGREDIENTS AND APPEARANCEProduct Information