Label: LIALDA- mesalamine tablet, delayed release
- NDC Code(s): 54092-476-01, 54092-476-02, 54092-476-08, 54092-476-12
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LIALDA safely and effectively. See full prescribing information for LIALDA. LIALDA® (mesalamine) delayed-release tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGELIALDA is indicated for the: induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis. treatment of mildly to moderately active ulcerative ...
-
2 DOSAGE AND ADMINISTRATIONAdministration Instructions - Evaluate renal function prior to initiation of LIALDA and periodically while on therapy. Swallow LIALDA tablets whole; do not split or crush. Administer LIALDA ...
-
3 DOSAGE FORMS AND STRENGTHSThe red-brown ellipsoidal delayed-release tablet containing 1.2 g mesalamine is debossed on one side and imprinted with S476.
-
4 CONTRAINDICATIONSLIALDA is contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or to any of the ingredients of LIALDA [see Warnings and Precautions (5.3), Adverse ...
-
5 WARNINGS AND PRECAUTIONS5.1 Renal Impairment - Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients given products such ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Renal impairment, including renal failure [see Warnings and Precautions (5.1)] Mesalamine-induced ...
-
7 DRUG INTERACTIONS7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs - The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs) ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Published data from meta-analyses, cohort studies, and case series on the use of mesalamine during pregnancy have not reliably informed an association with ...
-
10 OVERDOSAGELIALDA is an aminosalicylate, and symptoms of salicylate toxicity may include nausea, vomiting, abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness ...
-
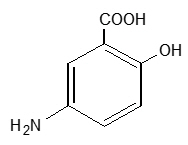
11 DESCRIPTIONEach LIALDA delayed-release tablet for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of mesalamine is not fully understood, but it appears to have a topical anti-inflammatory effect on the colonic epithelial cells. Mucosal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 104-week dietary carcinogenicity study in CD-1 mice, mesalamine at doses up to 2500 mg/kg/day was not ...
-
14 CLINICAL STUDIES14.1 Adults with Mildly to Moderately Active Ulcerative Colitis - Induction of Remission - Two similarly designed, randomized, double-blind, placebo-controlled trials (Study 1, NCT00503243 and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLIALDA is available as red-brown, ellipsoidal, film-coated, delayed-release tablets containing 1.2 g mesalamine, and debossed on one side imprinted with S476. NDC 54092-476-12 HDPE Bottle with a ...
-
17 PATIENT COUNSELING INFORMATIONRenal Impairment - Inform patients that LIALDA may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs, and periodic monitoring of ...
-
SPL UNCLASSIFIED SECTIONManufactured for Takeda Pharmaceuticals America, Inc., Lexington, MA 02421, USA by Cosmo S.p.A., Milan, Italy. LIALDA® is a registered trademark of Nogra Pharma Ltd., Ireland used under license. By ...
-
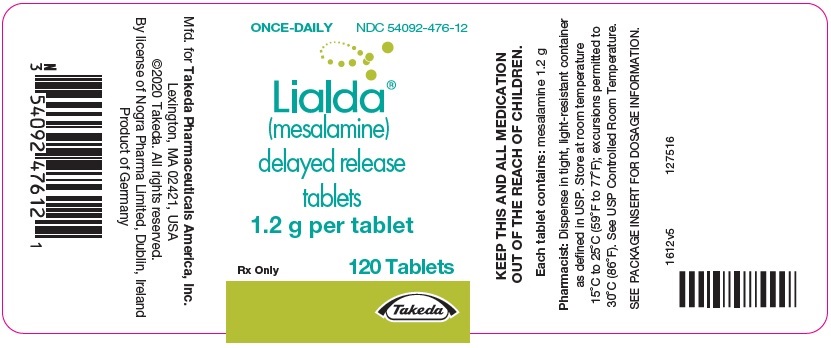
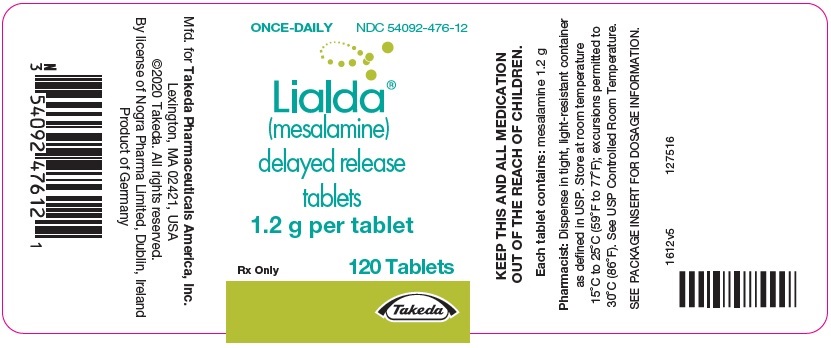
PRINCIPAL DISPLAY PANEL - 1.2 g Tablet Bottle LabelONCE-DAILY - NDC 54092-476-12 - Lialda® (mesalamine) delayed release - tablets - 1.2 g per tablet - Rx Only - 120 Tablets - Takeda
-
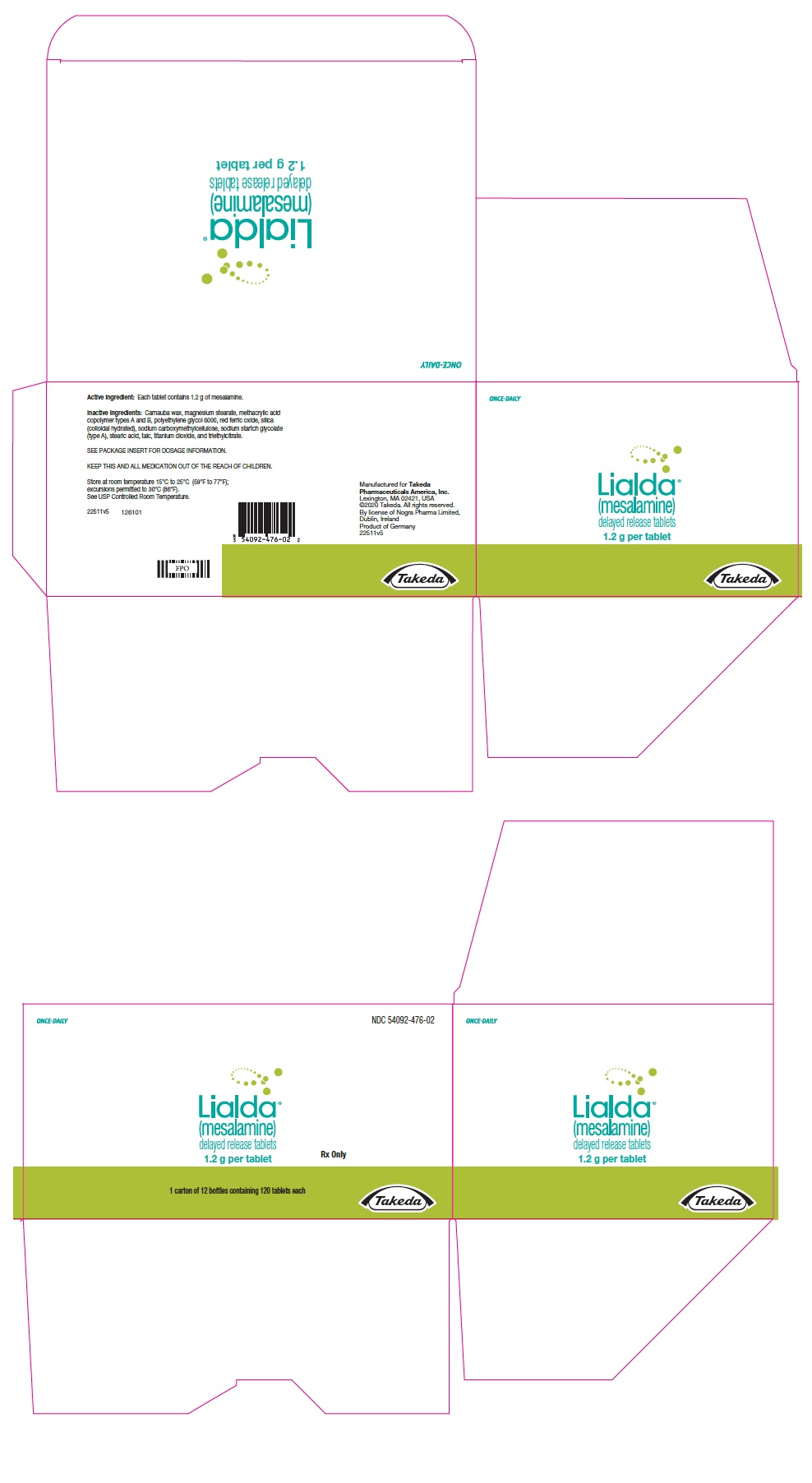
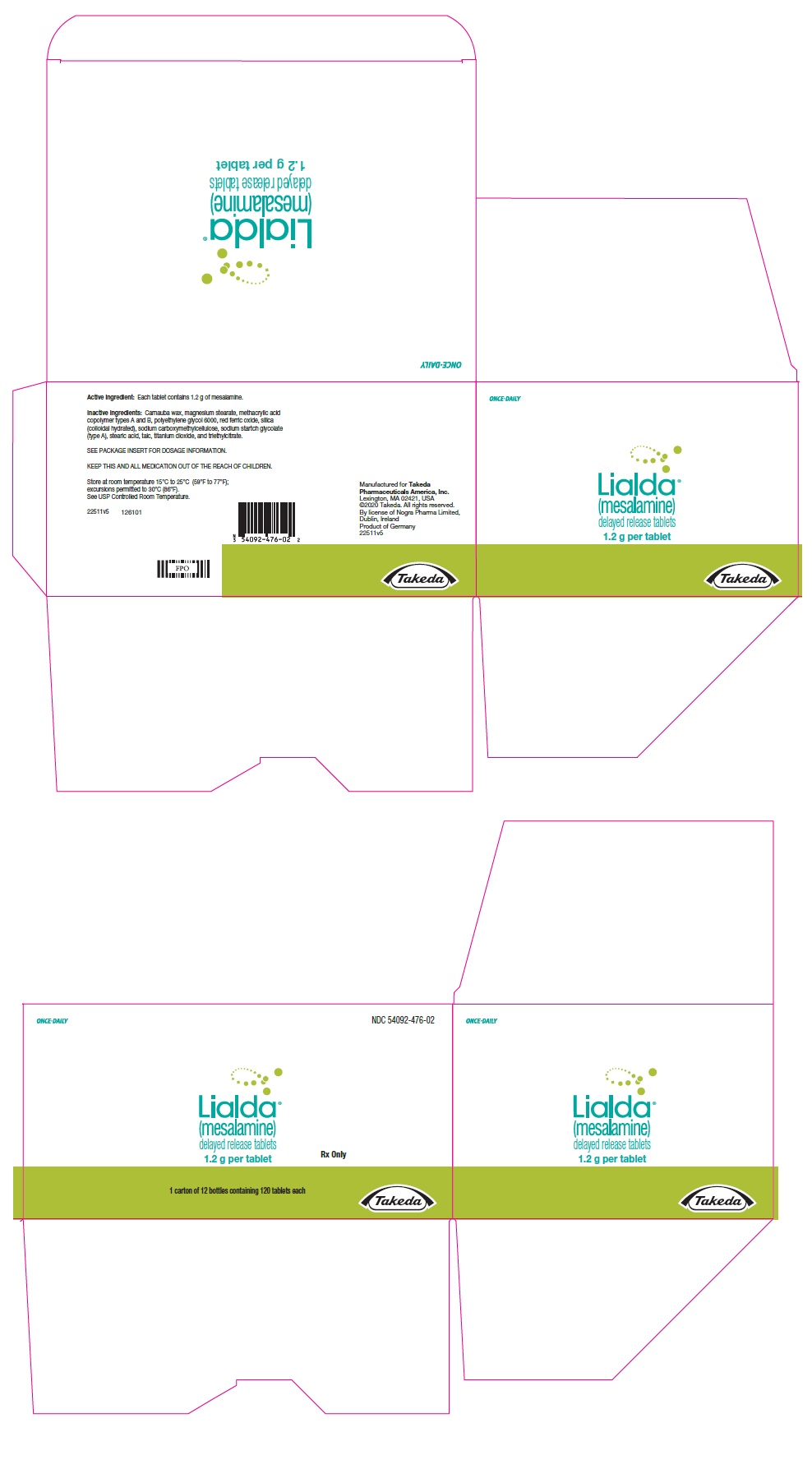
PRINCIPAL DISPLAY PANEL - 1.2 g Tablet Bottle CartonONCE-DAILY - NDC 54092-476-02 - Lialda® (mesalamine) delayed release tablets - 1.2 g per tablet - Rx Only - 1 carton of 12 bottles containing 120 tablets each - Takeda
-
INGREDIENTS AND APPEARANCEProduct Information