Label: FOSRENOL- lanthanum carbonate tablet, chewable
FOSRENOL- lanthanum carbonate powder
- NDC Code(s): 54092-252-45, 54092-252-90, 54092-253-15, 54092-253-90, view more
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FOSRENOL safely and effectively. See full prescribing information for FOSRENOL. FOSRENOL® (lanthanum carbonate) chewable tablets ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFOSRENOL® is a phosphate binder indicated to reduce serum phosphate in patients with end-stage renal disease (ESRD). Management of elevated serum phosphorus levels in patients with ESRD usually ...
-
2 DOSAGE AND ADMINISTRATIONDivide the total daily dose of FOSRENOL and take with or immediately after meals. The recommended initial total daily dose of FOSRENOL is 1,500 mg. Titrate the dose every 2 to 3 weeks until an ...
-
3 DOSAGE FORMS AND STRENGTHSFOSRENOL Chewable Tablets: 500 mg, 750 mg, and 1,000 mg. FOSRENOL Oral Powder: 750 mg and 1,000 mg.
-
4 CONTRAINDICATIONSContraindicated in patients with: - hypersensitivity to FOSRENOL or to any ingredient in the formulation. - bowel obstruction, including ileus and fecal impaction.
-
5 WARNINGS AND PRECAUTIONS5.1 Gastrointestinal Adverse Effects - Serious cases of gastrointestinal obstruction, ileus, subileus, gastrointestinal perforation, and fecal impaction have been reported in patients taking ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Gastrointestinal Adverse Effects [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Drugs Binding to Antacids - There is a potential for FOSRENOL to interact with compounds which bind to cationic antacids (i.e., aluminum-, magnesium-, or calcium-based); therefore, do not ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from case reports with use of FOSRENOL in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage ...
-
10 OVERDOSAGEThe symptoms associated with overdose are adverse reactions such as headache, nausea and vomiting. In clinical trials in healthy adults, gastrointestinal (GI) symptoms were reported with daily ...
-
11 DESCRIPTIONFOSRENOL contains lanthanum carbonate with molecular formula La2(CO3)3 xH2O (on average x=4-5 moles of water) and molecular weight 457.8 (anhydrous mass). Lanthanum carbonate is described as white ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - FOSRENOL is a phosphate binder that reduces absorption of phosphate by forming insoluble lanthanum phosphate complexes that pass through the GI tract unabsorbed. Both ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Oral administration of lanthanum carbonate to rats for up to 104 weeks, at doses up to 1,500 mg of the salt per kg/day (2.5 times the ...
-
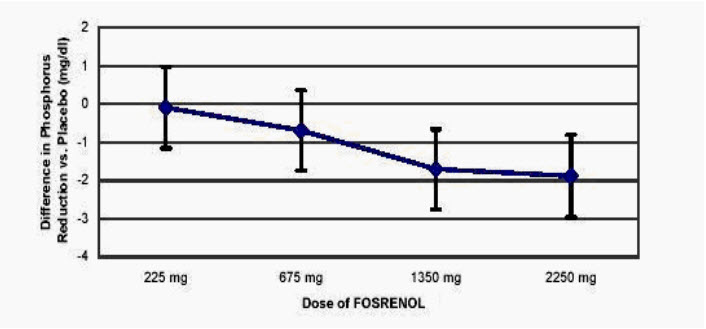
14 CLINICAL STUDIESThe effectiveness of FOSRENOL in reducing serum phosphorus in patients with ESRD was demonstrated in one short-term, placebo-controlled, double-blind dose-ranging study; two placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 FOSRENOL Chewable Tablets - FOSRENOL is supplied as a chewable tablet in three dosage strengths for oral administration: 500-mg tablets, 750-mg tablets, and 1,000-mg tablets. Each chewable ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Advise patients to take FOSRENOL with or immediately after meals [see Dosage and Administration (2)]. Instruct ...
-
SPL UNCLASSIFIED SECTIONFOSRENOL Chewable Tablets manufactured by Patheon Manufacturing Services LLC, 5900 Martin Luther King Jr. Highway - Greenville, NC 27834 - FOSRENOL Oral Powder manufactured by Catalent Germany ...
-
MEDICATION GUIDE FOSRENOL® (foss-wren-all) (lanthanum carbonate)Read this Medication Guide before you start taking FOSRENOL and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare ...
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle LabelNDC 54092-252-45 - FOSRENOL® (lanthanum carbonate) chewable tablets - 500 mg - Do not swallow tablets whole. Chew or crush tablets completely - before swallowing. Take with or immediately after ...
-
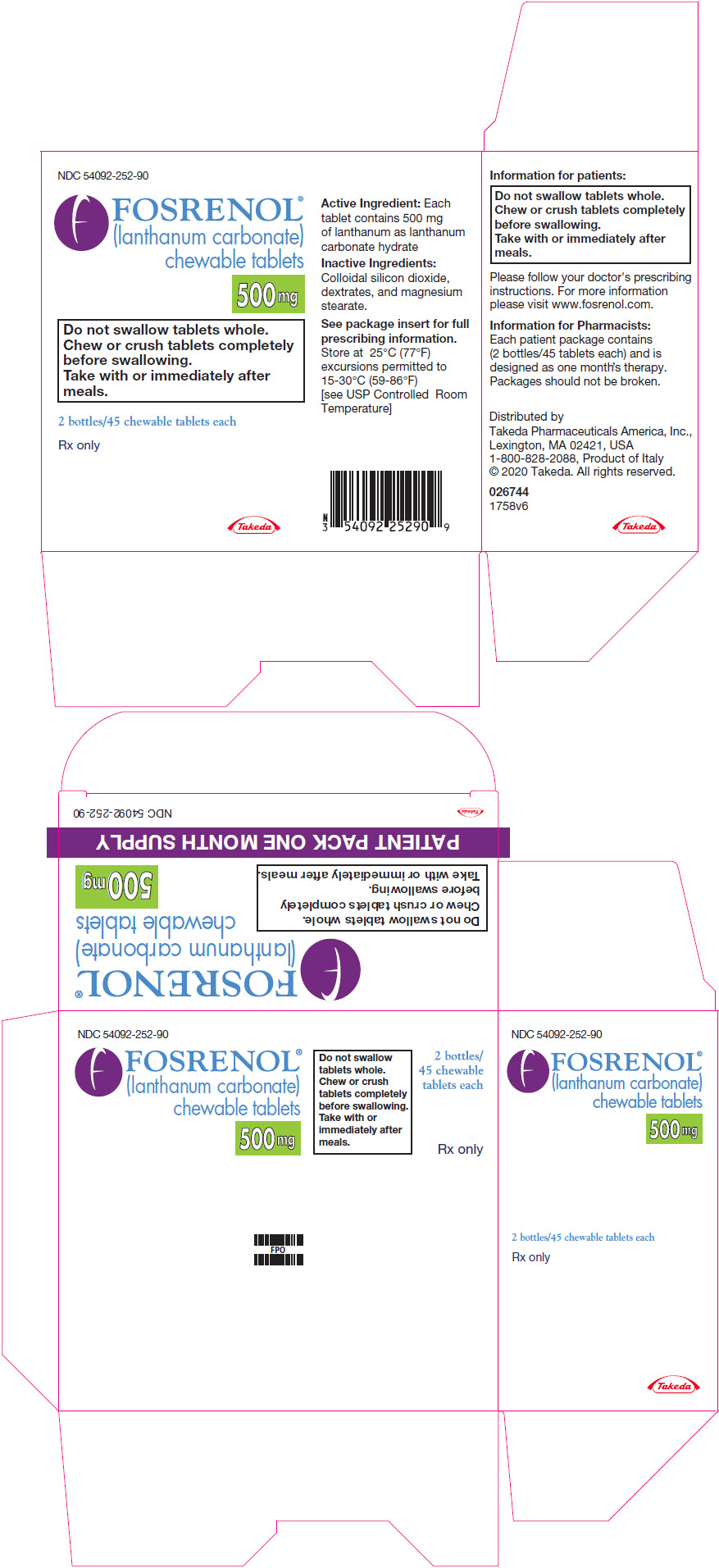
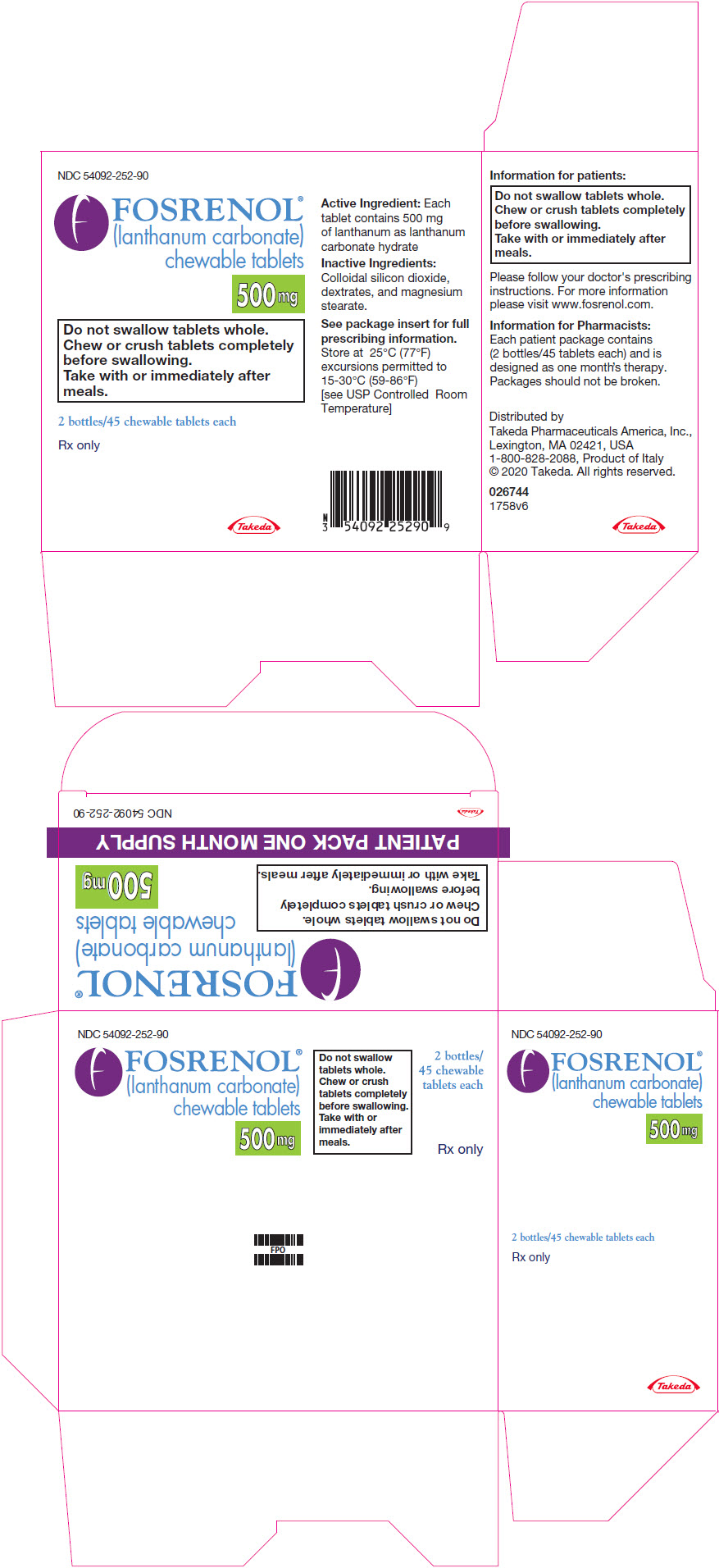
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle PackageNDC 54092-252-90 - FOSRENOL® (lanthanum carbonate) chewable tablets - 500 mg - Do not swallow tablets whole. Chew or crush tablets completely - before swallowing. Take with or immediately after - meals. 2 ...
-
PRINCIPAL DISPLAY PANEL - 750 mg Tablet Bottle LabelNDC 54092-253-15 - FOSRENOL® (lanthanum carbonate) chewable tablets - 750 mg - Do not swallow tablets whole. Chew or crush tablets completely - before swallowing. Take with or immediately after ...
-
PRINCIPAL DISPLAY PANEL - 750 mg Tablet Bottle PackageNDC 54092-253-90 - FOSRENOL® (lanthanum carbonate) chewable tablets - 750 mg - Do not swallow tablets whole. Chew or crush tablets completely - before swallowing. Take with or immediately after meals. 6 ...
-
PRINCIPAL DISPLAY PANEL - 1000 mg Tablet Bottle LabelNDC 54092-254-10 - FOSRENOL® (lanthanum carbonate) chewable tablets - 1000 mg - Do not swallow tablets whole. Chew or crush tablets completely - before swallowing. Take with or immediately after ...
-
PRINCIPAL DISPLAY PANEL - 1000 mg Tablet Bottle PackageNDC 54092-254-90 - FOSRENOL® (lanthanum carbonate) chewable tablets - 1000 mg - Do not swallow tablets whole. Chew or crush tablets completely - before swallowing. Take with or immediately after meals. 9 ...
-
PRINCIPAL DISPLAY PANEL - 750 mg Packet Carton - 10 Stick PackNDC 54092-256-01 - Fosrenol® (lanthanum carbonate) oral powder - 750 mg - Mix with small quantity of soft food - and take immediately - Takeda - 10 stick packs - Rx only
-
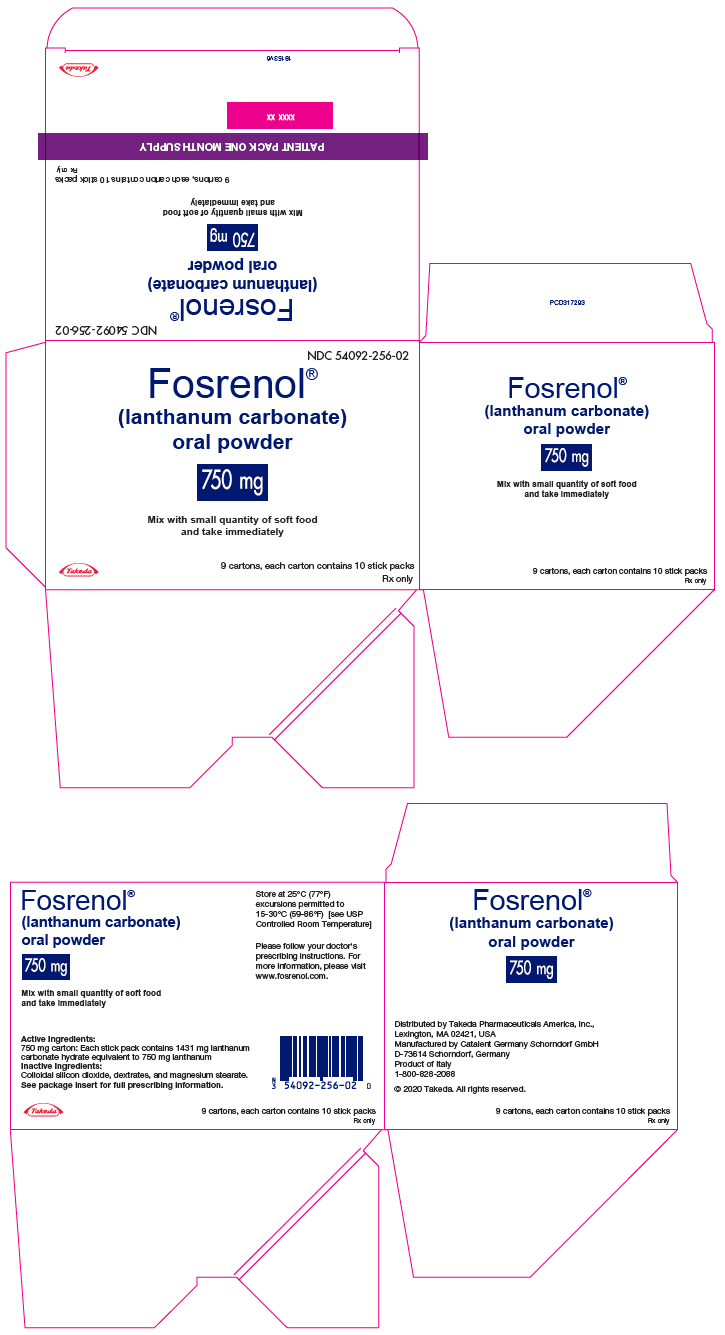
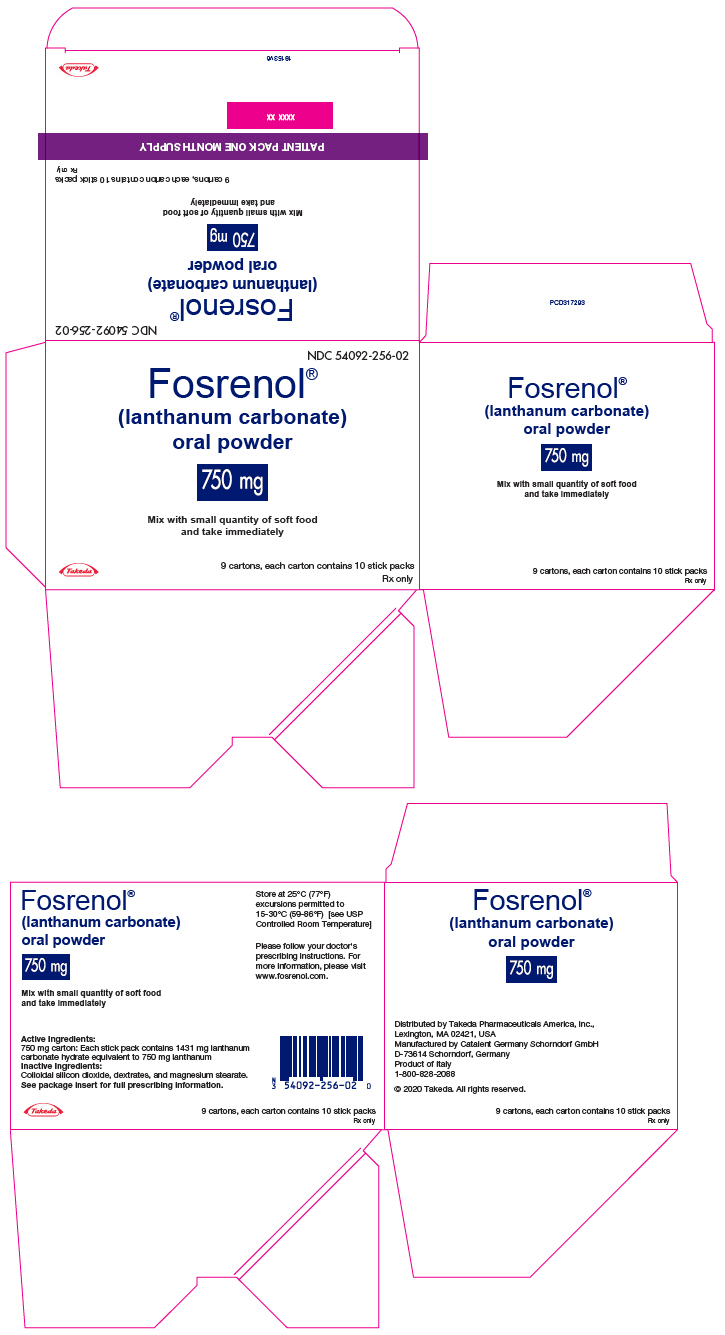
PRINCIPAL DISPLAY PANEL - 750 mg Packet Carton - 90 Stick PackNDC 54092-256-02 - Fosrenol® (lanthanum carbonate) oral powder - 750 mg - Mix with small quantity of soft food - and take immediately - Takeda - 9 cartons, each carton contains 10 stick packs - Rx ...
-
PRINCIPAL DISPLAY PANEL - 1000 mg Packet Carton - 10 Stick PackNDC 54092-257-01 - Fosrenol® (lanthanum carbonate) oral powder - 1000 mg - Mix with small quantity of soft food - and take immediately - Takeda - 10 stick packs - Rx only
-
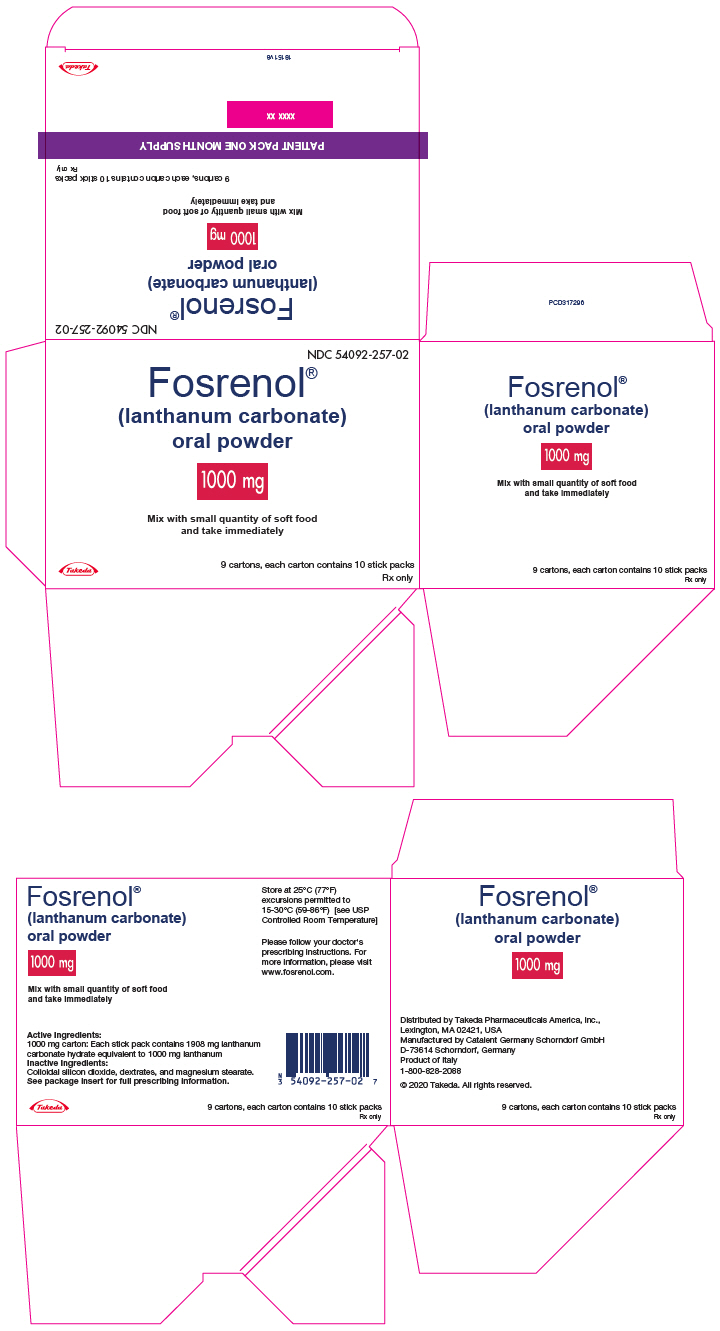
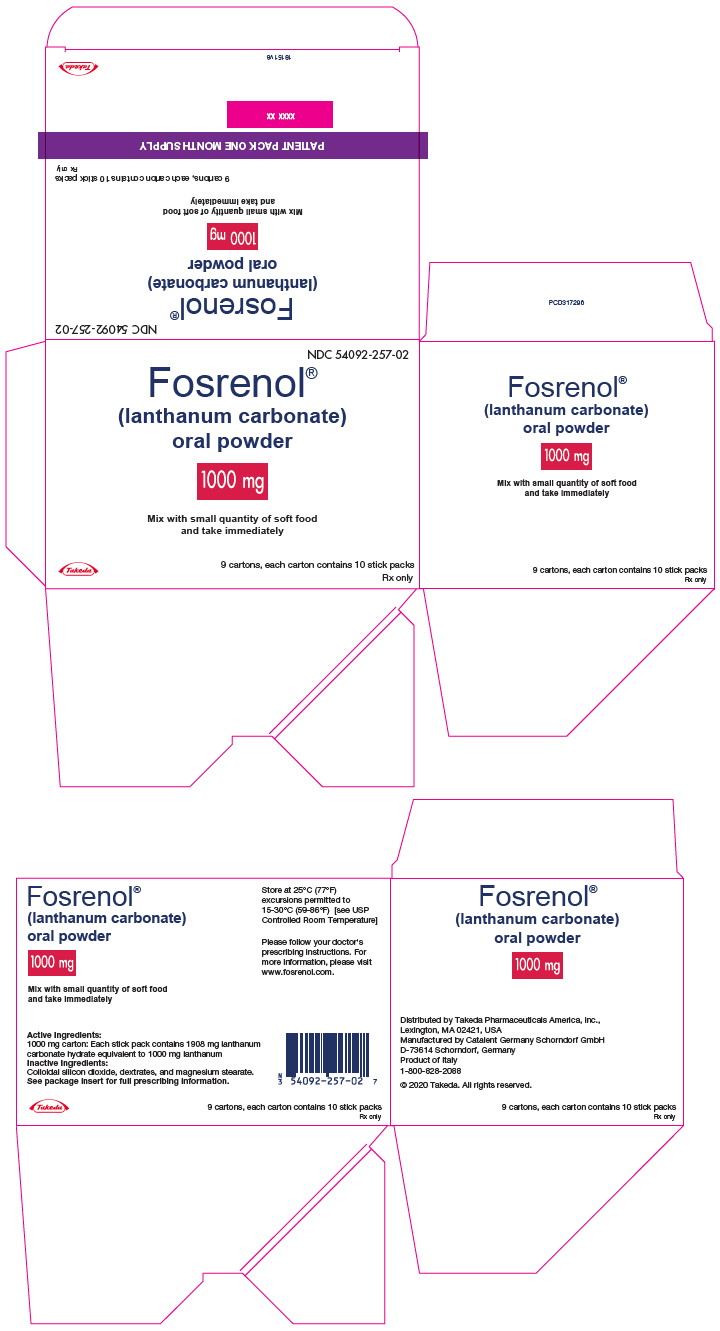
PRINCIPAL DISPLAY PANEL - 1000 mg Packet Carton - 90 Stick PackNDC 54092-257-02 - Fosrenol® (lanthanum carbonate) oral powder - 1000 mg - Mix with small quantity of soft food - and take immediately - Takeda - 9 cartons, each carton contains 10 stick packs - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information








 are registered trademarks of Takeda Pharmaceuticals U.S.A., Inc.
are registered trademarks of Takeda Pharmaceuticals U.S.A., Inc.