Label: SULINDAC tablet
-
NDC Code(s):
53489-478-01,
53489-478-02,
53489-478-03,
53489-478-05, view more53489-478-06, 53489-478-10, 53489-479-01, 53489-479-02, 53489-479-03, 53489-479-05, 53489-479-06, 53489-479-10
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use [see Warnings and Precautions].
- Sulindac tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see Contraindicationsand Warnings].
Gastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (see WARNINGS.)
-
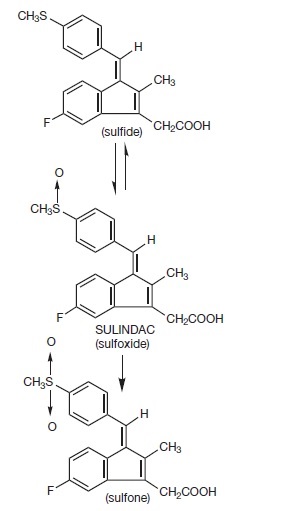
DESCRIPTIONSulindac is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)-5-fluoro-2-methyl-1-[[ p-(methylsulfinyl)phenyl]methylene]-1 - H-indene-3-acetic acid. It is not ...
Sulindac is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)-5-fluoro-2-methyl-1-[[ p-(methylsulfinyl)phenyl]methylene]-1 H-indene-3-acetic acid. It is not a salicylate, pyrazolone or propionic acid derivative. Its empirical formula is C 20H 17FO 3S, with a molecular weight of 356.42. Sulindac, a yellow crystalline compound, is a weak organic acid practically insoluble in water below pH 4.5, but very soluble as the sodium salt or in buffers of pH 6 or higher.
Sulindac tablets are available in 150 and 200 mg tablets for oral administration. Each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
Following absorption, sulindac undergoes two major biotransformations — reversible reduction to the sulfide metabolite, and irreversible oxidation to the sulfone metabolite. Available evidence indicates that the biological activity resides with the sulfide metabolite.
The structural formulas of sulindac and its metabolites are:
Close -
CLINICAL PHARMACOLOGYPharmacodynamics - Sulindac tablets are a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of ...
Pharmacodynamics
Sulindac tablets are a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Pharmacokinetics
Absorption
The extent of sulindac absorption from sulindac tablets is similar as compared to sulindac solution.
There is no information regarding food effect on sulindac absorption. Antacids containing magnesium hydroxide 200 mg and aluminum hydroxide 225 mg per 5 mL have been shown not to significantly decrease the extent of sulindac absorption.
TABLE 1 PHARMACOKINETIC
PARAMETERSNORMAL ELDERLY T max
Age 19–41 (n=24)
Age 65–87 (n=12) 400 mg qd
(200 mg tablet)
2.54 ± 1.52 S
5.75 ± 2.81 SF
3.38 ± 2.30 S
6.83 ± 4.19 SP
4.88 ± 2.57 SP
4.96 ± 2.36 SF
(150 mg tablet)
3.90 ± 2.30 S
5.85 ± 4.49 SP
6.15 ± 3.07 SF
Renal Clearance
(200 mg tablet)
68.12 ± 27.56 mL/min S
36.58 ± 12.61 mL/min SP
(150 mg tablet)
74.39 ± 34.15 mL/min S
41.75 ± 13.72 mL/min SP
Mean effective Half life (h)
7.8 S
16.4 SFS = Sulindac
SF = Sulindac Sulfide
SP = Sulindac SulfoneDistribution
Sulindac, and its sulfone and sulfide metabolites, are 93.1, 95.4, and 97.9% bound to plasma proteins, predominantly to albumin. Plasma protein binding measured over a concentration range (0.5–2.0 µg/mL) was constant. Following an oral, radiolabeled dose of sulindac in rats, concentrations of radiolabel in red blood cells were about 10% of those in plasma. Sulindac penetrates the blood-brain and placental barriers. Concentrations in brain did not exceed 4% of those in plasma. Plasma concentrations in the placenta and in the fetus were less than 25% and 5% respectively, of systemic plasma concentrations. Sulindac is excreted in rat milk; concentrations in milk were 10 to 20% of those levels in plasma. It is not known if sulindac is excreted in human milk.
Metabolism
Sulindac undergoes two major biotransformations of its sulfoxide moiety: oxidation to the inactive sulfone and reduction to the pharmacologically active sulfide. The latter is readily reversible in animals and in man. These metabolites are present as unchanged compounds in plasma and principally as glucuronide conjugates in human urine and bile. A dihydroxydihydro analog has also been identified as a minor metabolite in human urine.
With the twice-a-day dosage regimen, plasma concentrations of sulindac and its two metabolites accumulate: mean concentration over a dosage interval at steady state relative to the first dose averages 1.5 and 2.5 times higher, respectively, for sulindac and its active sulfide metabolite.
Sulindac and its sulfone metabolite undergo extensive enterohepatic circulation relative to the sulfide metabolite in animals. Studies in man have also demonstrated that recirculation of the parent drug sulindac and its sulfone metabolite is more extensive than that of the active sulfide metabolite. The active sulfide metabolite accounts for less than six percent of the total intestinal exposure to sulindac and its metabolites.
Biochemical as well as pharmacological evidence indicates that the activity of sulindac resides in its sulfide metabolite. An in-vitroassay for inhibition of cyclooxygenase activity exhibited an EC 50of 0.02 µM for sulindac sulfide. In-vivomodels of inflammation indicate that activity is more highly correlated with concentrations of the metabolite than with parent drug concentrations.
Elimination
Approximately 50% of the administered dose of sulindac is excreted in the urine with the conjugated sulfone metabolite accounting for the major portion. Less than 1% of the administered dose of sulindac appears in the urine as the sulfide metabolite. Approximately 25% is found in the feces, primarily as the sulfone and sulfide metabolites.
The mean effective half-life (T 1/2) is 7.8 and 16.4 hours, respectively, for sulindac and its active sulfide metabolite.
Because sulindac tablets are excreted in the urine primarily as biologically inactive forms, it may possibly affect renal function to a lesser extent than other non-steroidal anti-inflammatory drugs; however, renal adverse experiences have been reported with sulindac tablets (see ADVERSE REACTIONS).
In a study of patients with chronic glomerular disease treated with therapeutic doses of sulindac tablets, no effect was demonstrated on renal blood flow, glomerular filtration rate, or urinary excretion of prostaglandin E 2and the primary metabolite of prostacyclin, 6-keto-PGF 1α. However, in other studies in healthy volunteers and patients with liver disease, sulindac tablets were found to blunt the renal responses to intravenous furosemide, i.e., the diuresis, natriuresis, increments in plasma renin activity and urinary excretion of prostaglandins. These observations may represent a differentiation of the effects of sulindac tablets on renal functions based on differences in pathogenesis of the renal prostaglandin dependence associated with differing dose-response relationships of different NSAIDs to the various renal functions influenced by prostaglandins (see PRECAUTIONS).
In healthy men, the average fecal blood loss, measured over a two-week period during administration of 400 mg per day of sulindac tablets, was similar to that for placebo, and was statistically significantly less than that resulting from 4800 mg per day of aspirin.
Special Populations
Hepatic Insufficiency
Patients with acute and chronic hepatic disease may require reduced doses of sulindac tablets compared to patients with normal hepatic function since hepatic metabolism is an important elimination pathway.
Following a single dose, plasma concentrations of the active sulfide metabolite have been reported to be higher in patients with alcoholic liver disease compared to healthy normal subjects.
Renal Insufficiency
Sulindac pharmacokinetics have been investigated in patients with renal insufficiency. The disposition of sulindac was studied in end-stage renal disease patients requiring hemodialysis. Plasma concentrations of sulindac and its sulfone metabolite were comparable to those of normal healthy volunteers whereas concentrations of the active sulfide metabolite were significantly reduced. Plasma protein binding was reduced and the AUC of the unbound sulfide metabolite was about half that in healthy subjects.
Sulindac and its metabolites are not significantly removed from the blood in patients undergoing hemodialysis.
Since sulindac tablets are eliminated primarily by the kidneys, patients with significantly impaired renal function should be closely monitored.
A lower daily dosage should be anticipated to avoid excessive drug accumulation.
In controlled clinical studies sulindac tablets were evaluated in the following five conditions:
1. Osteoarthritis
In patients with osteoarthritis of the hip and knee, the anti-inflammatory and analgesic activity of sulindac tablets was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; improvement in ARA Functional Class; relief of night pain; improvement in overall evaluation of pain, including pain on weight bearing and pain on active and passive motion; improvement in joint mobility, range of motion, and functional activities; decreased swelling and tenderness; and decreased duration of stiffness following prolonged inactivity.
In clinical studies in which dosages were adjusted according to patient needs, sulindac tablets 200 to 400 mg daily were shown to be comparable in effectiveness to aspirin 2400 to 4800 mg daily. Sulindac tablets were generally well tolerated, and patients on it had a lower overall incidence of total adverse effects, of milder gastrointestinal reactions, and of tinnitus than did patients on aspirin. (See ADVERSE REACTIONS. )
2. Rheumatoid arthritis
In patients with rheumatoid arthritis, the anti-inflammatory and analgesic activity of sulindac tablets was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; reduction in overall joint pain; reduction in duration and severity of morning stiffness; reduction in day and night pain; decrease in time required to walk 50 feet; decrease in general pain as measured on a visual analog scale; improvement in the Ritchie articular index; decrease in proximal interphalangeal joint size; improvement in ARA Functional Class; increase in grip strength; reduction in painful joint count and score; reduction in swollen joint count and score; and increased flexion and extension of the wrist.
In clinical studies in which dosages were adjusted according to patient needs, sulindac tablets 300 to 400 mg daily were shown to be comparable in effectiveness to aspirin 3600 to 4800 mg daily. Sulindac tablets were generally well tolerated, and patients on it had a lower overall incidence of total adverse effects, of milder gastrointestinal reactions, and of tinnitus than did patients on aspirin. (See ADVERSE REACTIONS. )
In patients with rheumatoid arthritis, sulindac tablets may be used in combination with gold salts at usual dosage levels. In clinical studies, sulindac tablets added to the regimen of gold salts usually resulted in additional symptomatic relief but did not alter the course of the underlying disease.
3. Ankylosing spondylitis
In patients with ankylosing spondylitis, the anti-inflammatory and analgesic activity of sulindac tablets was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; improvement in ARA Functional Class; improvement in patient and investigator evaluation of spinal pain, tenderness and/or spasm; reduction in the duration of morning stiffness; increase in the time to onset of fatigue; relief of night pain; increase in chest expansion; and increase in spinal mobility evaluated by fingers-to-floor distance, occiput to wall distance, the Schober Test, and the Wright Modification of the Schober Test. In a clinical study in which dosages were adjusted according to patient need, sulindac tablets 200 to 400 mg daily were as effective as indomethacin 75 to 150 mg daily. In a second study, sulindac tablets 300 to 400 mg daily were comparable in effectiveness to phenylbutazone 400 to 600 mg daily. Sulindac tablets were better tolerated than phenylbutazone. (See ADVERSE REACTIONS. )
4. Acute painful shoulder (Acute subacromial bursitis/supraspinatus tendinitis)
In patients with acute painful shoulder (acute subacromial bursitis/supraspinatus tendinitis), the anti-inflammatory and analgesic activity of sulindac tablets was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; relief of night pain, spontaneous pain, and pain on active motion; decrease in local tenderness; and improvement in range of motion measured by abduction, and internal and external rotation. In clinical studies in acute painful shoulder, sulindac tablets 300 to 400 mg daily and oxyphenbutazone 400 to 600 mg daily were shown to be equally effective and well tolerated.
Close5 . Acute gouty arthritis
In patients with acute gouty arthritis, the anti-inflammatory and analgesic activity of sulindac tablets was demonstrated by clinical measurements that included: assessments by both the patient and investigator of overall response; relief of weight-bearing pain; relief of pain at rest and on active and passive motion; decrease in tenderness; reduction in warmth and swelling; increase in range of motion; and improvement in ability to function. In clinical studies, sulindac tablets at 400 mg daily and phenylbutazone at 600 mg daily were shown to be equally effective. In these short-term studies in which reduction of dosage was permitted according to response, both drugs were equally well tolerated.
-
INDICATIONS AND USAGECarefully consider the potential benefits and risks of sulindac tablets and other treatment options before deciding to use sulindac tablets. Use the lowest effective dose for the shortest duration ...
Carefully consider the potential benefits and risks of sulindac tablets and other treatment options before deciding to use sulindac tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
Sulindac tablets are indicated for acute or long-term use in the relief of signs and symptoms of the following:
- Osteoarthritis
- Rheumatoid arthritis*
- Ankylosing spondylitis
- Acute painful shoulder (Acute subacromial bursitis/supraspinatus tendinitis)
- Acute gouty arthritis
-
CONTRAINDICATIONSSulindac tablets are contraindicated in patients with known hypersensitivity to sulindac or the excipients (see - DESCRIPTION). Sulindac tablets should not be given to patients who have ...
Sulindac tablets are contraindicated in patients with known hypersensitivity to sulindac or the excipients (see DESCRIPTION).
Sulindac tablets should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic/anaphylactoid reactions to NSAIDs have been reported in such patients (see WARNINGS – Anaphylactic/Anaphylactoid Reactions, and PRECAUTIONS – Preexisting Asthma).
- In the setting of coronary artery bypass graft (CABG) surgery [see WARNINGS].
-
WARNINGSCARDIOVASCULAR EFFECTS - Cardiovascular Thrombotic Events - Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of ...Close
CARDIOVASCULAR EFFECTS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as sulindac, increases the risk of serious gastrointestinal (GI) events [see Warnings].
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10–14 days following CABG surgery found an increased incidence of myocardial infarction and stroke. NSAIDs are contraindicated in the setting of CABG [see Contraindications].
Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.
Avoid the use of sulindac tablets in patients with a recent MI unless the benefits are expected to outweigh the risk of recurrent CV thrombotic events. If sulindac tablets are used in patients with a recent MI, monitor patients for signs of cardiac ischemia.
Hypertension
NSAIDs, including sulindac tablets, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including sulindac tablets, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Heart Failure and Edema
The Coxib and traditional NSAID Trialists’ Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of sulindac may blunt the CV effects of several therapeutic agents used to treat these medical conditions [e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers (ARBs)] [see Drug Interactions].
Avoid the use of sulindac tablets in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If sulindac tablets are used in patients with sever heart failure, monitor patients for signs of worsening heart failure.
Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including sulindac tablets, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3–6 months, and in about 2–4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleedingwho use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Hepatic Effects
In addition to hypersensitivity reactions involving the liver, in some patients the findings are consistent with those of cholestatic hepatitis (see WARNINGS, Hypersensitivity). As with other non-steroidal anti-inflammatory drugs, borderline elevations of one or more liver tests without any other signs and symptoms may occur in up to 15% of patients taking NSAIDs including sulindac tablets. These laboratory abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. The SGPT (ALT) test is probably the most sensitive indicator of liver dysfunction. Meaningful (3 times the upper limit of normal) elevations of SGPT or SGOT (AST) occurred in controlled clinical trials in less than 1% of patients. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with sulindac tablets. Although such reactions as described above are rare, if abnormal liver tests persist or worsen, if clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), sulindac tablets should be discontinued.
In clinical trials with sulindac tablets, the use of doses of 600 mg/day has been associated with an increased incidence of mild liver test abnormalities (see DOSAGE AND ADMINISTRATIONfor maximum dosage recommendation).
* The safety and effectiveness of sulindac tablets have not been established in rheumatoid arthritis patients who are designated in the American Rheumatism Association classification as Functional Class IV (incapacitated, largely or wholly bedridden, or confined to wheelchair; little or no self-care).
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a non-steroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, patients who are volume-depleted, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of sulindac tablets in patients with advanced renal disease. Therefore, treatment with sulindac tablets is not recommended in these patients with advanced renal disease. If sulindac tablets therapy must be initiated, close monitoring of the patient's renal function is advisable.
Anaphylactic/Anaphylactoid Reactions
As with other NSAIDs, anaphylactic/anaphylactoid reactions may occur in patients without known prior exposure to sulindac tablets. Sulindac tablets should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONSand PRECAUTIONS – Preexisting Asthma) . Emergency help should be sought in cases where an anaphylactic/anaphylactoid reaction occurs.
Serious Skin Reactions
NSAIDs, including sulindac, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. NSAIDs can also cause fixed drug eruption (FDE). FDE may present as a more severe variant known as generalized bullous fixed drug eruption (GBFDE), which can be life-threatening. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions, and to discontinue the use of sulindac tablets at the first appearance of skin rash or any other sign of hypersensitivity. Sulindac tablets are contraindicated in patients with previous serious skin reactions to NSAIDs (see CONTRAINDICATIONS).
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as sulindac tablets. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue sulindac tablets and evaluate the patient immediately .
Hypersensitivity
Rarely, fever and other evidence of hypersensitivity (see ADVERSE REACTIONS) including abnormalities in one or more liver function tests and severe skin reactions have occurred during therapy with sulindac tablets. Fatalities have occurred in these patients. Hepatitis, jaundice, or both, with or without fever, may occur usually within the first one to three months of therapy. Determinations of liver function should be considered whenever a patient on therapy with sulindac tablets develops unexplained fever, rash or other dermatologic reactions or constitutional symptoms. If unexplained fever or other evidence of hypersensitivity occurs, therapy with sulindac tablets should be discontinued. The elevated temperature and abnormalities in liver function caused by sulindac tablets characteristically have reverted to normal after discontinuation of therapy. Administration of sulindac tablets should not be reinstituted in such patients.
Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs, including sulindac tablets, in pregnant women at about 30 weeks gestation and later. NSAIDs including sulindac tablets, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.
Oligohydramnios/Neonatal Renal Impairment:
Use of NSAIDs, including sulindac tablets, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation.
Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some postmarketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.
If NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit sulindac tablets use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if sulindac tablets treatment extends beyond 48 hours. Discontinue sulindac tablets if oligohydramnios occurs and follow up according to clinical practice (see PRECAUTIONS; Pregnancy).
-
PRECAUTIONSGeneral - Sulindac tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease ...
General
Sulindac tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of sulindac tablets in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including sulindac tablets. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including sulindac tablets, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving sulindac tablets who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other non-steroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, sulindac tablets should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Renal Calculi
Sulindac metabolites have been reported rarely as the major or a minor component in renal stones in association with other calculus components. Sulindac tablets should be used with caution in patients with a history of renal lithiasis, and they should be kept well hydrated while receiving sulindac tablets.
Pancreatitis
Pancreatitis has been reported in patients receiving sulindac tablets (see ADVERSE REACTIONS). Should pancreatitis be suspected, the drug should be discontinued and not restarted, supportive medical therapy instituted, and the patient monitored closely with appropriate laboratory studies (e.g., serum and urine amylase, amylase/creatinine clearance ratio, electrolytes, serum calcium, glucose, lipase, etc.). A search for other causes of pancreatitis as well as those conditions which mimic pancreatitis should be conducted.
Ocular Effects
Because of reports of adverse eye findings with non-steroidal anti-inflammatory agents, it is recommended that patients who develop eye complaints during treatment with sulindac tablets have ophthalmologic studies.
Hepatic Insufficiency
In patients with poor liver function, delayed, elevated and prolonged circulating levels of the sulfide and sulfone metabolites may occur. Such patients should be monitored closely; a reduction of daily dosage may be required.
SLE and Mixed Connective Tissue Disease
In patients with systemic lupus erythematosus (SLE) and mixed connective tissue disease, there may be an increased risk of aseptic meningitis (see ADVERSE REACTIONS).
Information for Patients
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
- Cardiovascular Thrombotic Events
- Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their healthcare provider immediately [see Warnings].
- Sulindac tablets, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).
- Serious Skin Reactions, including DRESS
- Advise patients to stop taking sulindac tablets immediately if they develop any type of rash or fever and to contact their healthcare provider as soon as possible (see WARNINGS).
- Heart Failure and Edema
- Advise patients to be alert for the symptoms of congestive heart failure including shortness of
- breath, unexplained weight gain, or edema and to contact their healthcare provider if such
- symptoms occur [see WARNINGS].
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
- Patients should be informed of the signs of an anaphylactic/anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).
- Fetal Toxicity
- Inform pregnant women to avoid use of sulindac tablets and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with sulindac tablets is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours (see WARNINGS; Fetal Toxicity, PRECAUTIONS; Pregnancy).
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, sulindac tablets should be discontinued.
Drug Interactions
ACE-Inhibitors and Angiotensin II Antagonists
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors and angiotensin II antagonists. These interactions should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors or angiotensin II antagonists. In some patients with compromised renal function (e.g., elderly patients or patients who are volume-depleted, including those on diuretic therapy) who are being treated with non-steroidal anti-inflammatory drugs, the co-administration of an NSAID and an ACE-inhibitor or an angiotensin II antagonist may result in further deterioration of renal function, including possible acute renal failure, which is usually reversible. Therefore, monitor renal function periodically in patients receiving ACEIs or AIIAs and NSAIDs in combination therapy.
Acetaminophen
Acetaminophen had no effect on the plasma levels of sulindac or its sulfide metabolite.
Aspirin
The concomitant administration of aspirin with sulindac significantly depressed the plasma levels of the active sulfide metabolite. A double-blind study compared the safety and efficacy of sulindac tablets 300 or 400 mg daily given alone or with aspirin 2.4 g/day for the treatment of osteoarthritis. The addition of aspirin did not alter the types of clinical or laboratory adverse experiences for sulindac tablets; however, the combination showed an increase in the incidence of gastrointestinal adverse experiences. Since the addition of aspirin did not have a favorable effect on the therapeutic response to sulindac tablets, the combination is not recommended.
Cyclosporine
Administration of non-steroidal anti-inflammatory drugs concomitantly with cyclosporine has been associated with an increase in cyclosporine-induced toxicity, possibly due to decreased synthesis of renal prostacyclin. NSAIDs should be used with caution in patients taking cyclosporine, and renal function should be carefully monitored.
Diflunisal
The concomitant administration of sulindac tablets and diflunisal in normal volunteers resulted in lowering of the plasma levels of the active sulindac sulfide metabolite by approximately one-third.
Diuretics
Clinical studies, as well as post marketing observations, have shown that sulindac tablets can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS, Renal Effects) , as well as to assure diuretic efficacy.
DMSO
DMSO should not be used with sulindac. Concomitant administration has been reported to reduce the plasma levels of the active sulfide metabolite and potentially reduce efficacy. In addition, this combination has been reported to cause peripheral neuropathy.
Lithium
NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
NSAIDs
The concomitant use of sulindac tablets with other NSAIDs is not recommended due to the increased possibility of gastrointestinal toxicity, with little or no increase in efficacy.
Oral anticoagulants
Although sulindac and its sulfide metabolite are highly bound to protein, studies in which sulindac tablets were given at a dose of 400 mg daily have shown no clinically significant interaction with oral anticoagulants. However, patients should be monitored carefully until it is certain that no change in their anticoagulant dosage is required. Special attention should be paid to patients taking higher doses than those recommended and to patients with renal impairment or other metabolic defects that might increase sulindac blood levels. The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
Oral hypoglycemic agents
Although sulindac and its sulfide metabolite are highly bound to protein, studies in which sulindac tablets were given at a dose of 400 mg daily, have shown no clinically significant interaction with oral hypoglycemic agents. However, patients should be monitored carefully until it is certain that no change in their hypoglycemic dosage is required. Special attention should be paid to patients taking higher doses than those recommended and to patients with renal impairment or other metabolic defects that might increase sulindac blood levels.
Probenecid
Probenecid given concomitantly with sulindac had only a slight effect on plasma sulfide levels, while plasma levels of sulindac and sulfone were increased. Sulindac was shown to produce a modest reduction in the uricosuric action of probenecid, which probably is not significant under most circumstances.
Pregnancy
Risk Summary
Use of NSAIDs, including sulindac tablets, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of sulindac tablets use between about 20 and 30 weeks of gestation, and avoid sulindac tablets use at about 30 weeks of gestation and later in pregnancy (see WARNINGS; Fetal Toxicity).
Premature Closure of Fetal Ductus Arteriosus
Use of NSAIDs, including sulindac tablets, at about 30 weeks gestation or later in pregnancy increases
the risk of premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal
renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.
Data from observational studies regarding other potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive. In animal reproduction studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. In reproduction studies in the rat, a decrease in average fetal weight and an increase in numbers of dead pups were observed on the first day of the postpartum period at dosage levels of 20 and 40 mg/kg/day (2½ and 5 times the usual maximum daily dose in humans), although there was no adverse effect on the survival and growth during the remainder of the postpartum period. Sulindac prolongs the duration of gestation in rats, as do other compounds of this class. Visceral and skeletal malformations observed in low incidence among rabbits in some teratology studies did not occur at the same dosage levels in repeat studies, nor at a higher dosage level in the same species. However, animal reproduction studies are not always predictive of human response. Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as sulindac, resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including sulindac tablets, can cause premature closure of the fetal ductus arteriosus (see WARNINGS; Fetal Toxicity).
Oligohydramnios/Neonatal Renal Impairment
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If sulindac tablets treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue sulindac tablets and follow up according to clinical practice (see WARNINGS; Fetal Toxicity).
Data
- Human Data
There are no adequate and well controlled studies in pregnant women. Sulindac should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus. The known effects of drugs of this class on the human fetus during the third trimester of pregnancy include: constriction of the ductus arteriosus prenatally, tricuspid incompetence, and pulmonary hypertension; non-closure of the ductus arteriosus postnatally which may be resistant to medical management; myocardial degenerative changes, platelet dysfunction with resultant bleeding, intracranial bleeding, renal dysfunction or failure, renal injury/dysgenesis which may result in prolonged or permanent renal failure, oligohydramnios, gastrointestinal bleeding or perforation, and increased risk of necrotizing enterocolitis.
- Premature Closure of Fetal Ductus Arteriosus:
- Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
- Oligohydramnios/Neonatal Renal Impairment:
- Published studies and postmarketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
- Methodological limitations of these postmarketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Labor and Delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of sulindac tablets on labor and delivery in pregnant women are unknown.
Nursing Mothers
It is not known whether this drug is excreted in human milk; however, it is secreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from sulindac tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
CloseGeriatric Use
As with any NSAID, caution should be exercised in treating the elderly (65 years and older) since advancing age appears to increase the possibility of adverse reactions. Elderly patients seem to tolerate ulceration or bleeding less well than other individuals and many spontaneous reports of fatal GI events are in this population (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).
Sulindac tablets are known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function (see WARNINGS, Renal Effects).
-
ADVERSE REACTIONSThe following adverse reactions were reported in clinical trials or have been reported since the drug was marketed. The probability exists of a causal relationship between sulindac tablets and ...
The following adverse reactions were reported in clinical trials or have been reported since the drug was marketed. The probability exists of a causal relationship between sulindac tablets and these adverse reactions. The adverse reactions which have been observed in clinical trials encompass observations in 1,865 patients, including 232 observed for at least 48 weeks.
Incidence Greater Than 1%
Gastrointestinal
The most frequent types of adverse reactions occurring with sulindac tablets are gastrointestinal; these include gastrointestinal pain (10%), dyspepsia**, nausea** with or without vomiting, diarrhea**, constipation**, flatulence, anorexia and gastrointestinal cramps.
Dermatologic
Rash**, pruritus.
Central Nervous System
Dizziness**, headache**, nervousness.
Special Senses
Tinnitus.
Miscellaneous
Edema (see WARNINGS).
Incidence Less Than 1 in 100
Gastrointestinal
Gastritis, gastroenteritis or colitis. Peptic ulcer and gastrointestinal bleeding have been reported. GI perforation and intestinal strictures (diaphragms) have been reported rarely.
Liver function abnormalities; jaundice, sometimes with fever; cholestasis; hepatitis; hepatic failure.
There have been rare reports of sulindac metabolites in common bile duct "sludge" and in biliary calculi in patients with symptoms of cholecystitis who underwent a cholecystectomy.
Pancreatitis (see PRECAUTIONS).
Ageusia; glossitis.
Dermatologic
Stomatitis, sore or dry mucous membranes, alopecia, photosensitivity.
Erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, fixed drug eruption (FDE), and exfoliative dermatitis have been reported.
Cardiovascular
Congestive heart failure, especially in patients with marginal cardiac function; palpitation; hypertension.
Hematologic
Thrombocytopenia; ecchymosis; purpura; leukopenia; agranulocytosis; neutropenia; bone marrow depression, including aplastic anemia; hemolytic anemia; increased prothrombin time in patients on oral anticoagulants (see PRECAUTIONS).
Genitourinary
Urine discoloration; dysuria; vaginal bleeding; hematuria; proteinuria; crystalluria; renal impairment, including renal failure; interstitial nephritis; nephrotic syndrome.
Renal calculi containing sulindac metabolites have been observed rarely.
Metabolic
Hyperkalemia.
Musculoskeletal
Muscle weakness.
Psychiatric
Depression; psychic disturbances including acute psychosis.
Nervous System
Vertigo; insomnia; somnolence; paresthesia; convulsions; syncope; aseptic meningitis (especially in patients with systemic lupus erythematosus (SLE) and mixed connective tissue disease, see PRECAUTIONS).
Special Senses
Blurred vision; visual disturbances; decreased hearing; metallic or bitter taste.
Respiratory
Epistaxis.
Hypersensitivity Reactions
Anaphylaxis; angioneurotic edema; urticaria; bronchial spasm; dyspnea.
Hypersensitivity vasculitis.
A potentially fatal apparent hypersensitivity syndrome has been reported. This syndrome may include constitutional symptoms (fever, chills, diaphoresis, flushing), cutaneous findings (rash or other dermatologic reactions — see above), conjunctivitis, involvement of major organs (changes in liver function including hepatic failure, jaundice, pancreatitis, pneumonitis with or without pleural effusion, leukopenia, leukocytosis, eosinophilia, disseminated intravascular coagulation, anemia, renal impairment, including renal failure), and other less specific findings (adenitis, arthralgia, arthritis, myalgia, fatigue, malaise, hypotension, chest pain, tachycardia).
CloseCausal Relationship Unknown
A rare occurrence of fulminant necrotizing fasciitis, particularly in association with Group A β-hemolytic streptococcus, has been described in persons treated with non-steroidal anti-inflammatory agents, sometimes with fatal outcome (see also PRECAUTIONS, General).
Other reactions have been reported in clinical trials or since the drug was marketed, but occurred under circumstances where a causal relationship could not be established. However, in these rarely reported events, that possibility cannot be excluded. Therefore, these observations are listed to serve as alerting information to physicians.
Cardiovascular
Arrhythmia.
Metabolic
Hyperglycemia.
Nervous System
Neuritis.
Special Senses
Disturbances of the retina and its vasculature.
Miscellaneous
Gynecomastia.
-
MANAGEMENT OF OVERDOSAGECases of overdosage have been reported and rarely, deaths have occurred. The following signs and symptoms may be observed following overdosage: stupor, coma, diminished urine output and ...
Cases of overdosage have been reported and rarely, deaths have occurred. The following signs and symptoms may be observed following overdosage: stupor, coma, diminished urine output and hypotension.
In the event of overdosage, the stomach should be emptied by inducing vomiting or by gastric lavage, and the patient carefully observed and given symptomatic and supportive treatment.
Animal studies show that absorption is decreased by the prompt administration of activated charcoal and excretion is enhanced by alkalinization of the urine.
Close -
DOSAGE AND ADMINISTRATIONCarefully consider the potential benefits and risks of sulindac tablets and other treatment options before deciding to use sulindac tablets. Use the lowest effective dose for the shortest duration ...
Carefully consider the potential benefits and risks of sulindac tablets and other treatment options before deciding to use sulindac tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with sulindac tablets, the dose and frequency should be adjusted to suit an individual patient's needs.
Sulindac tablets should be administered orally twice a day with food. The maximum dosage is 400 mg per day. Dosages above 400 mg per day are not recommended.
In osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, the recommended starting dosage is 150 mg twice a day. The dosage may be lowered or raised depending on the response.
A prompt response (within one week) can be expected in about one-half of patients with osteoarthritis, ankylosing spondylitis, and rheumatoid arthritis. Others may require longer to respond.
In acute painful shoulder (acute subacromial bursitis/supraspinatus tendinitis) and acute gouty arthritis, the recommended dosage is 200 mg twice a day. After a satisfactory response has been achieved, the dosage may be reduced according to the response. In acute painful shoulder, therapy for 7–14 days is usually adequate. In acute gouty arthritis, therapy for 7 days is usually adequate.
**Incidence between 3% and 9%. Those reactions occurring in 1% to 3% of patients are not marked with an asterisk.
Close -
HOW SUPPLIEDSulindac tablets USP are supplied as follows: Sulindac tablets, 150 mg, yellow, round, unscored, debossed MP 112 - Bottles of 50 - NDC 53489-478-02 - Bottles of 60 - NDC ...
Sulindac tablets USP are supplied as follows:
Sulindac tablets, 150 mg, yellow, round, unscored, debossed MP 112
Bottles of 50
NDC 53489-478-02
Bottles of 60
NDC 53489-478-06
Bottles of 100
NDC 53489-478-01
Bottles of 250
NDC 53489-478-03
Bottles of 500
NDC 53489-478-05
Bottles of 1000
NDC 53489-478-10
Sulindac tablets, 200 mg, yellow, round, scored, debossed MP 116
Bottles of 50
NDC 53489-479-02
Bottles of 60
NDC 53489-479-06
Bottles of 100
NDC 53489-479-01
Bottles of 250
NDC 53489-479-03
Bottles of 500
NDC 53489-479-05
Bottles of 1000
NDC 53489-479-10
CloseStore at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature]DISPENSE IN TIGHT, LIGHT-RESISTANT CONTAINER.
-
SPL UNCLASSIFIED SECTIONDistributed by: Sun Pharmaceutical Industries, Inc. Cranbury, NJ 08512 - Rev 13, October 2024
-
MEDICATION GUIDEMedication Guide - for - Nonsteroidal Anti-inflammatory Drugs (NSAIDs) What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs ...
Medication Guide
for
Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?
NSAIDs can cause serious side effects, including:
- Increased risk of a heart attack or stroke that can lead to death.This risk may happen early in treatment and may increase:
- with increasing doses of NSAIDs
- with longer use of NSAIDs
- Do not take NSAIDs right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
- Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.
- Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:
- anytime during use
- without warning symptoms
- that may cause death
The risk of getting an ulcer or bleeding increases with:
- past history of stomach ulcers, or stomach or intestinal bleeding with use of NSAIDs
- taking medicines called "corticosteroids", "anti-coagulants", "SSRIs", or "SNRIs"
- increasing doses of NSAIDs
- longer use of NSAIDs
- smoking
- drinking alcohol
- older age
- poor health
- advanced liver disease
- bleeding problems
- NSAIDs should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain.
Who should not take NSAIDs?
Do not take NSAIDs:
- if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs.
- right before or after heart bypass surgery.
Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems
- have high blood pressure
- have asthma
- are pregnant or plan to become pregnant. Taking NSAIDs at about 20 weeks of pregnancy or later may harm your unborn baby. If you need to take NSAIDs for more than 2 days when you are between 20 and 30 weeks of pregnancy, your healthcare provider may need to monitor the amount of fluid in your womb around your baby. You should not take NSAIDs after about 30 weeks of pregnancy.
- are breastfeeding or plan to breast feed.
Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements.NSAIDs and some other medicines can interact with each other and cause serious side effects. Do not start taking any new medicine without talking to your healthcare provider first.
- What are the possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including:
See “What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?
- new or worse high blood pressure
- heart failure
- liver problems including liver failure
- kidney problems including kidney failure
- low red blood cells (anemia)
- life-threatening skin reactions
- life-threatening allergic reactions
- Other side effects of NSAIDs include: stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness.
- Get emergency help right away if you get any of the following symptoms:
- shortness of breath or trouble breathing
- chest pain
- weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
- Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms:
- nausea
- more tired or weaker than usual
- diarrhea
- itching
- your skin or eyes look yellow
- indigestion or stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- skin rash or blisters with fever
- swelling of the arms, legs, hands and feet
- If you take too much of your NSAID, call your healthcare provider or get medical help right away.
- These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs.
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about NSAIDs
- Aspirin is an NSAID but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some NSAIDs are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by: Sun Pharmaceutical Industries, Inc.
- Cranbury, NJ 08512
Rev 02, November 2020
Close -
PRINCIPAL DISPLAY PANEL - 150 mg Tablet Bottle Label

-
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label

-
INGREDIENTS AND APPEARANCEProduct Information
SULINDAC sulindac tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53489-478 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULINDAC (UNII: 184SNS8VUH) (SULINDAC - UNII:184SNS8VUH) SULINDAC 150 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color yellow Score no score Shape ROUND Size 9mm Flavor Imprint Code MP;112 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53489-478-02 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 2 NDC:53489-478-06 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 3 NDC:53489-478-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 4 NDC:53489-478-03 250 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 5 NDC:53489-478-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 6 NDC:53489-478-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072050 09/04/2009 SULINDAC sulindac tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53489-479 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULINDAC (UNII: 184SNS8VUH) (SULINDAC - UNII:184SNS8VUH) SULINDAC 200 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color yellow Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code MP;116 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53489-479-02 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 2 NDC:53489-479-06 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 3 NDC:53489-479-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 4 NDC:53489-479-03 250 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 5 NDC:53489-479-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 6 NDC:53489-479-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/04/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072051 09/04/2009 Labeler - Sun Pharmaceutical Industries, Inc. (146974886)
CloseEstablishment Name Address ID/FEI Business Operations Frontida BioPharm Inc. 080243260 manufacture(53489-478, 53489-479) , analysis(53489-478, 53489-479) , pack(53489-478, 53489-479)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
SULINDAC tablet
Number of versions: 14
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Oct 25, 2024 | 16 (current) | download |
| Aug 2, 2024 | 15 | download |
| May 13, 2021 | 14 | download |
| Apr 19, 2021 | 13 | download |
| Feb 27, 2018 | 12 | download |
| May 23, 2017 | 11 | download |
| Jan 6, 2015 | 10 | download |
| Jun 7, 2011 | 9 | download |
| Apr 22, 2011 | 8 | download |
| Apr 21, 2011 | 7 | download |
| Aug 18, 2010 | 6 | download |
| Sep 18, 2009 | 4 | download |
| Jul 14, 2009 | 3 | download |
| Feb 5, 2008 | 1 | download |
Get Label RSS Feed for this Drug
SULINDAC tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=a47e4259-d44b-4b7b-b517-841cb6840982
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
SULINDAC tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 53489-478-01 |
| 2 | 53489-478-02 |
| 3 | 53489-478-03 |
| 4 | 53489-478-05 |
| 5 | 53489-478-06 |
| 6 | 53489-478-10 |
| 7 | 53489-479-01 |
| 8 | 53489-479-02 |
| 9 | 53489-479-03 |
| 10 | 53489-479-05 |
| 11 | 53489-479-06 |
| 12 | 53489-479-10 |