Label: SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE tablet
- NDC Code(s): 53489-144-01, 53489-144-05, 53489-144-10
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
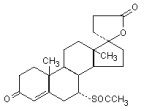
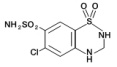
DESCRIPTIONSpironolactone and hydrochlorothiazide oral tablets contain: spironolactone . . . . . . . . . . . . . . . . 25 mg - hydrochlorothiazide . . . . . . . . . . . . 25 mg - Spironolactone, an aldosterone ...
-
ACTIONS / CLINICAL PHARMACOLOGYMechanism of action: Spironolactone and hydrochlorothiazide tablets are a combination of two diuretic agents with different but complementary mechanisms and sites of action, thereby providing ...
-
INDICATIONS AND USAGESpironolactone, an ingredient of spironolactone and hydrochlorothiazide tablets, has been shown to be a tumorigen in chronic toxicity studies in rats (see Precautions section). Spironolactone and ...
-
CONTRAINDICATIONSSpironolactone and hydrochlorothiazide tablets are contraindicated in patients with anuria, acute renal insufficiency, significant impairment of renal excretory function, hypercalcemia ...
-
WARNINGSPotassium supplementation, either in the form of medication or as a diet rich in potassium, should not ordinarily be given in association with spironolactone and hydrochlorothiazide tablets ...
-
PRECAUTIONSGeneral: Serum Electrolyte Abnormalities: Spironolactone can cause hyperkalemia. The risk of hyperkalemia may be increased in patients with renal insufficiency, diabetes mellitus or with ...
-
ADVERSE REACTIONSThe following adverse reactions have been reported and, within each category (body system), are listed in order of decreasing severity. Hydrochlorothiazide: Body as a whole ...
-
OVERDOSAGEThe oral LD50 of spironolactone is greater than 1000 mg/kg in mice, rats, and rabbits. The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats. Acute overdosage of ...
-
DOSAGE AND ADMINISTRATIONOptimal dosage should be established by individual titration of the components. Edema in adults (congestive heart failure, hepatic cirrhosis, or nephrotic syndrome). The usual maintenance dose ...
-
HOW SUPPLIEDSpironolactone and hydrochlorothiazide tablets, USP are supplied as follows: Spironolactone and hydrochlorothiazide tablets, 25 mg/25 mg are buff, round, unscored, debossed MP 40. Bottles of ...
-
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label

-
INGREDIENTS AND APPEARANCEProduct Information