Label: BACLOFEN tablet
- NDC Code(s): 52817-318-10, 52817-319-10, 52817-320-00, 52817-320-10, view more
- Packager: TruPharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
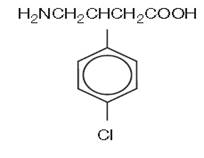
DESCRIPTIONBaclofen, USP is a muscle relaxant and antispastic. Its chemical name is 4-amino-3-(4-chlorophenyl)-butanoic acid. The structural formula is: C - 10H - 12ClNO - 2M.W. 213.66 - Baclofen ...
-
CLINICAL PHARMACOLOGYThe precise mechanism of action of baclofen is not fully known. Baclofen is capable of inhibiting both monosynaptic and polysynaptic reflexes at the spinal level, possibly by hyperpolarization of ...
-
INDICATIONS AND USAGEBaclofen tablets, USP are useful for the alleviation of signs and symptoms of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain ...
-
CONTRAINDICATIONSHypersensitivity to baclofen.
-
WARNINGSa. Neonatal Withdrawal Symptoms:Withdrawal symptoms have been reported starting hours to days after delivery in neonates whose mothers were treated with oral baclofen throughout pregnancy. The ...
-
PRECAUTIONSBecause of the possibility of sedation, patients should be cautioned regarding the operation of automobiles or other dangerous machinery, and activities made hazardous by decreased alertness ...
-
ADVERSE REACTIONSThe most common is transient drowsiness (10 to 63%). In one controlled study of 175 patients, transient drowsiness was observed in 63% of those receiving baclofen compared to 36% of those in the ...
-
OVERDOSAGESigns and Symptoms:Vomiting, muscular hypotonia, drowsiness, accommodation disorders, coma, respiratory depression and seizures. Treatment:In the alert patient, empty the stomach promptly by ...
-
DOSAGE AND ADMINISTRATIONThe determination of optimal dosage requires individual titration. Start therapy at a low dosage and increase gradually until optimum effect is achieved (usually between 40-80 mg daily). The ...
-
HOW SUPPLIEDBaclofen Tablets, USP 5 mg are available as a White to off white, round flat-faced, beveled edge tablets debossed with "023" on one side and plain on other side, containing 5 mg baclofen USP ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-319-10) 100 Tablets - Baclofen Tablets, USP 5 mg - Rx only - Rubicon Research Private Limited
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-320-10) 100 Tablets - Baclofen Tablets, USP 10 mg - Rx only - Rubicon Research Private Limited
-
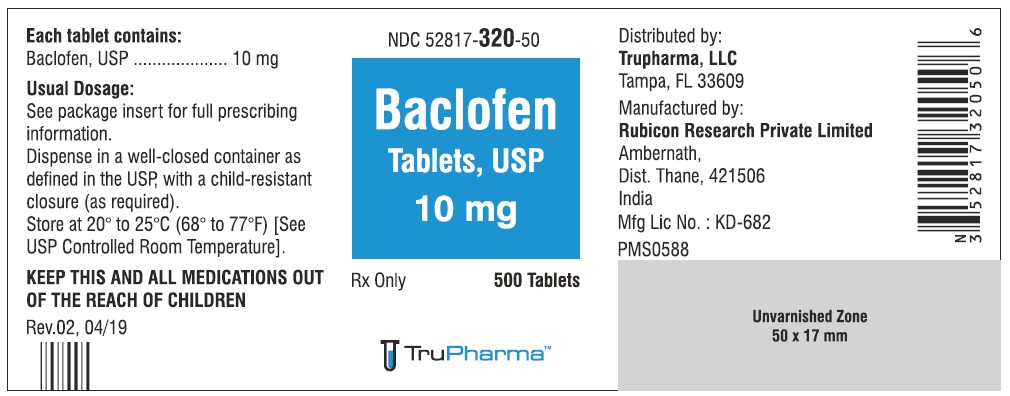
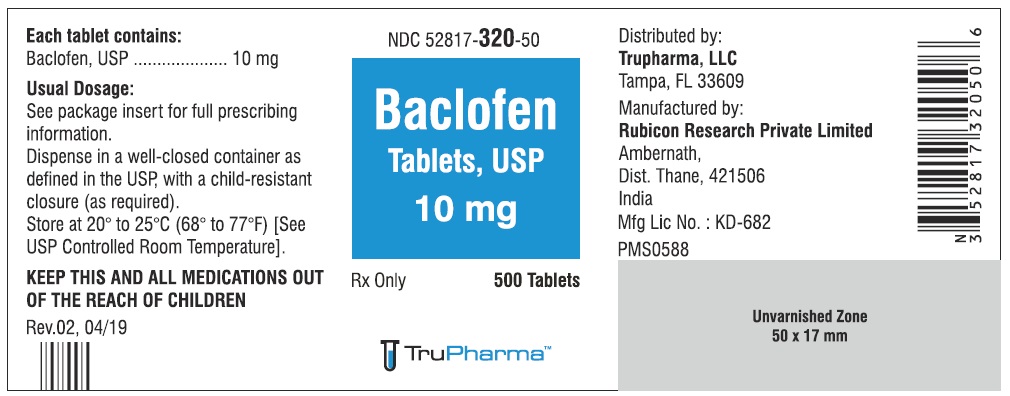
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-320-50) 500 Tablets - Baclofen Tablets, USP 10 mg - Rx only - Rubicon Research Private Limited
-
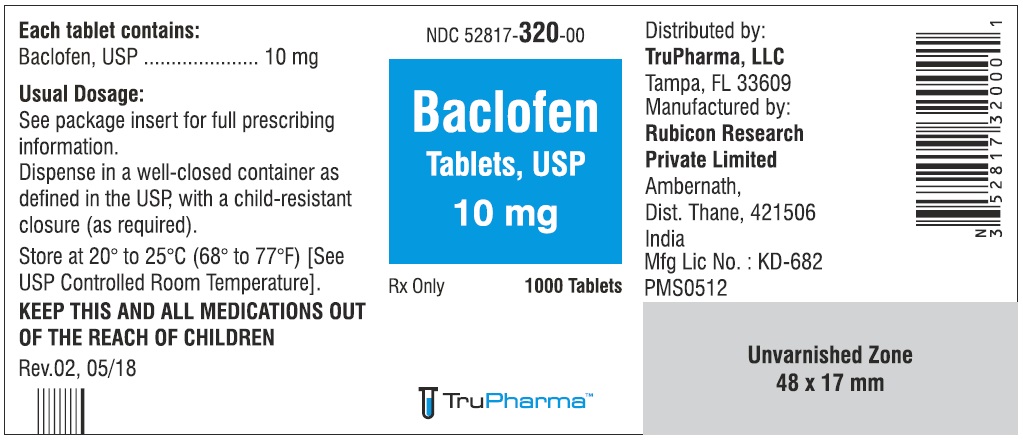
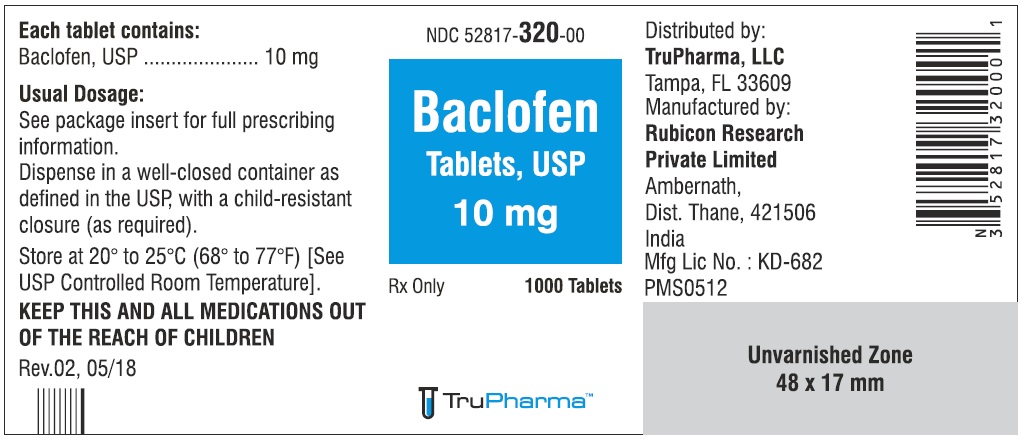
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-320-00) 1000 Tablets - Baclofen Tablets, USP 10 mg - Rx only - Rubicon Research Private Limited
-
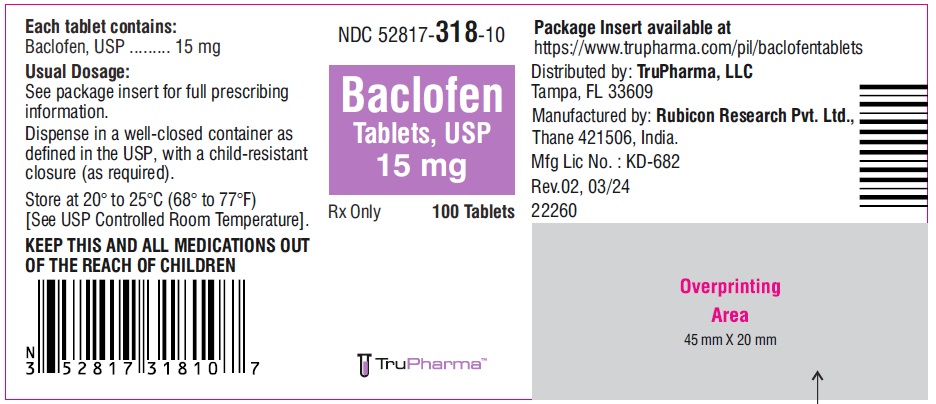
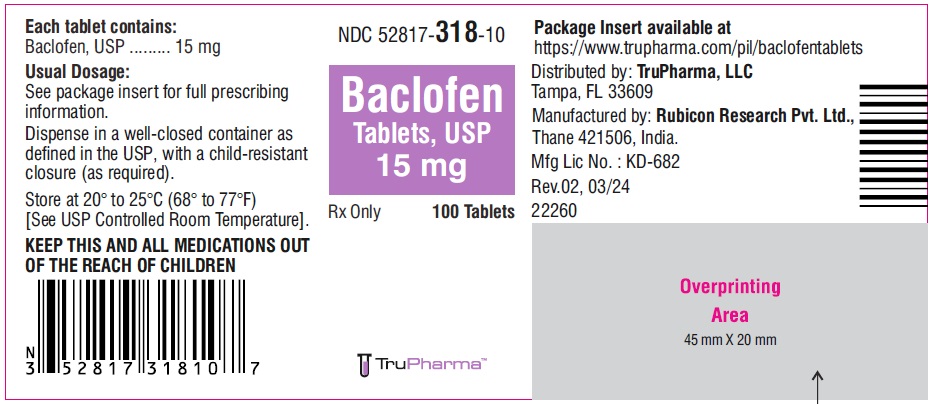
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-318-10) 100 Tablets - Baclofen Tablets, USP 15 mg - Rx only - Rubicon Research Private Limited
-
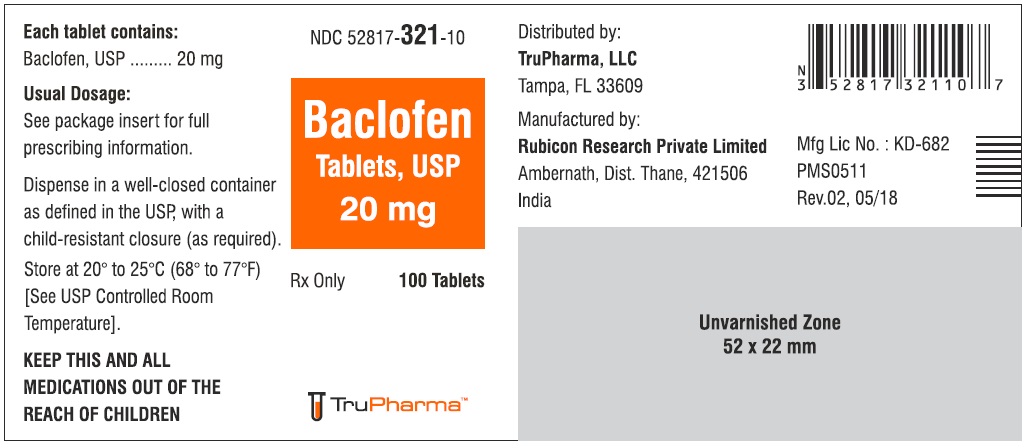
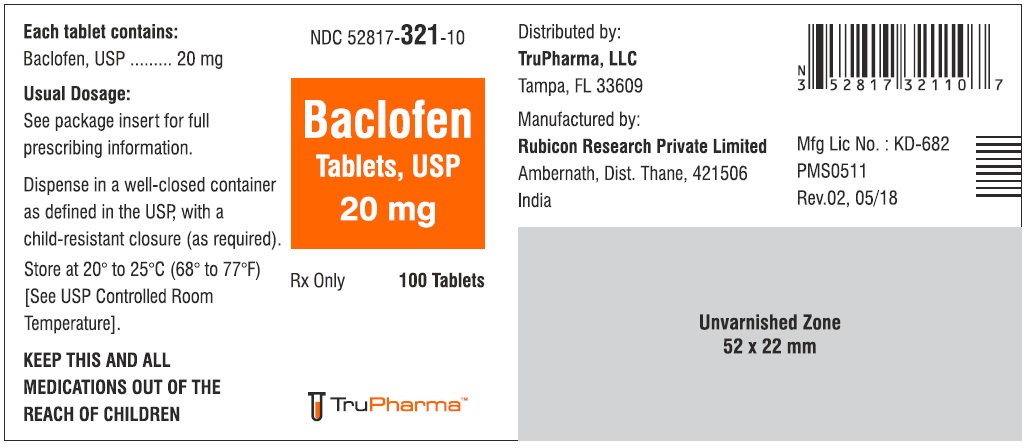
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-321-10) 100 Tablets - Baclofen Tablets, USP 20 mg - Rx only - Rubicon Research Private Limited
-
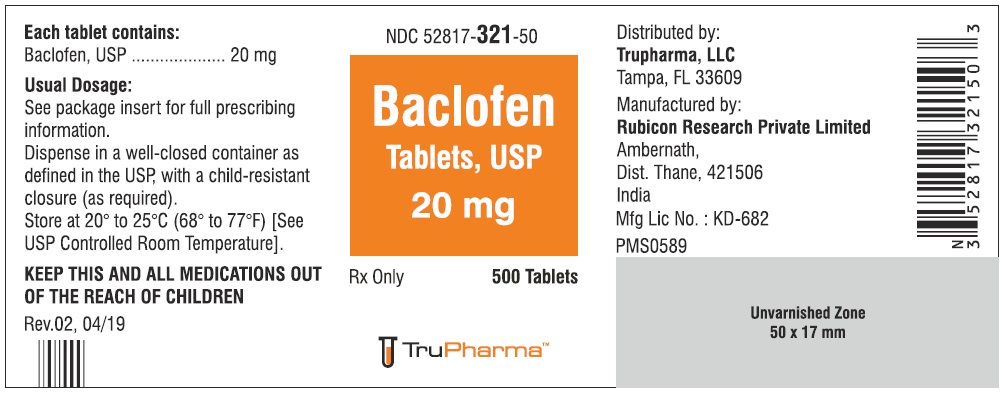
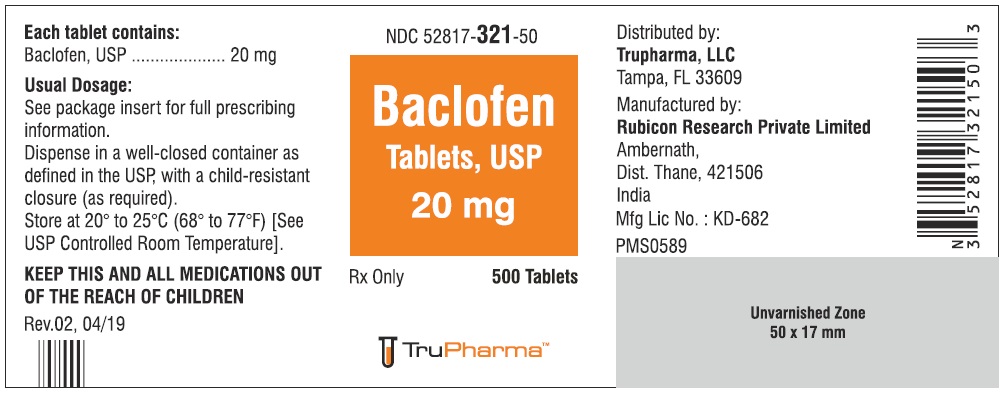
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-321-50) 500 Tablets - Baclofen Tablets, USP 20 mg - Rx only - Rubicon Research Private Limited
-
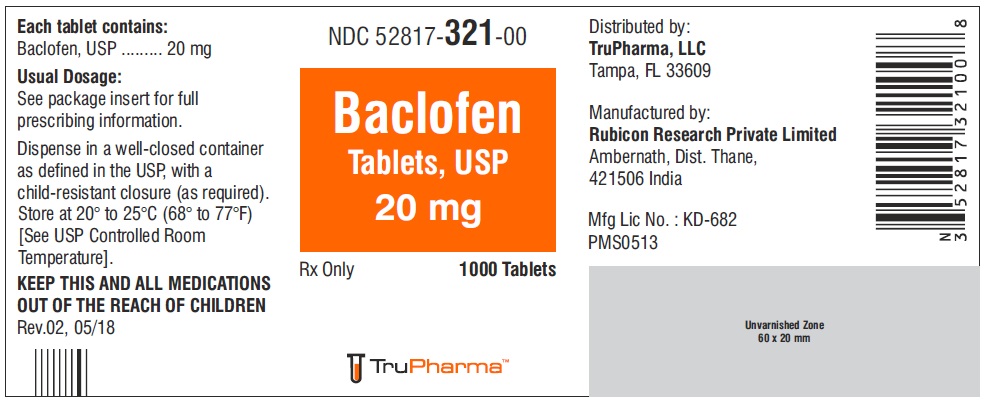
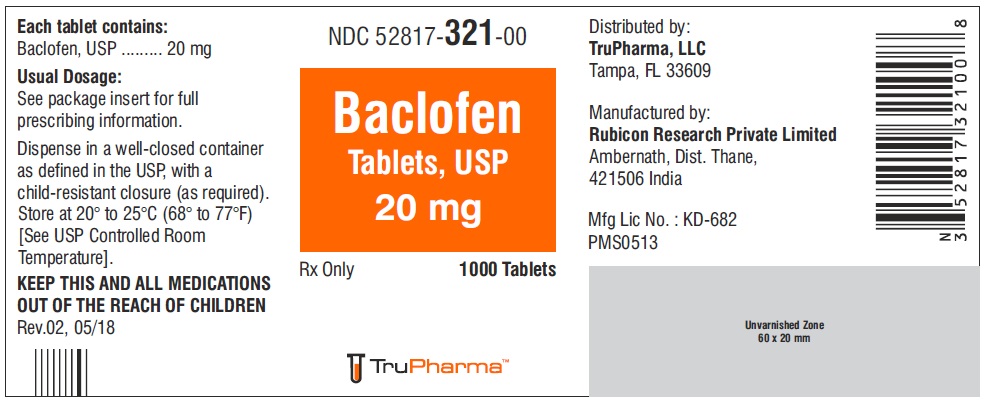
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC ( 52817-321-00) 1000 Tablets - Baclofen Tablets, USP 20 mg - Rx only - Rubicon Research Private Limited
-
INGREDIENTS AND APPEARANCEProduct Information