Label: ERYTHROMYCIN tablet, delayed release
- NDC Code(s): 52536-180-03, 52536-180-10, 52536-183-03, 52536-183-10, view more
- Packager: Wilshire Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONENTERIC-COATED - Rx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Erythromycin Delayed-Release Tablets and other antibacterial drugs, Erythromycin ...
-
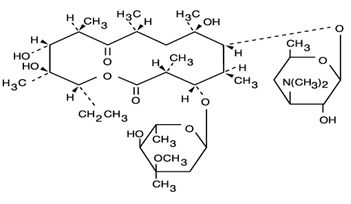
DESCRIPTIONErythromycin Delayed-Release Tablets, USP are an antibacterial product containing erythromycin base in a specially enteric-coated tablet. The coating protects the antibiotic from the inactivating ...
-
CLINICAL PHARMACOLOGYOrally administered erythromycin base and its salts are readily absorbed in the microbiologically active form. Interindividual variations in the absorption of erythromycin are, however, observed ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of Erythromycin Delayed-Release Tablets and other antibacterial drugs, Erythromycin Delayed-Release Tablets ...
-
CONTRAINDICATIONSErythromycin is contraindicated in patients with known hypersensitivity to this antibiotic. Erythromycin is contraindicated in patients taking terfenadine, astemizole, cisapride, pimozide ...
-
WARNINGSHepatotoxicity - There have been reports of hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, occurring in ...
-
PRECAUTIONSGeneral - Prescribing Erythromycin Delayed-Release Tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the ...
-
ADVERSE REACTIONSThe most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. They include nausea, vomiting, abdominal pain, diarrhea and anorexia. Symptoms of ...
-
OVERDOSAGEIn case of overdosage, erythromycin should be discontinued. Overdosage should be handled with the prompt elimination of unabsorbed drug and all other appropriate measures should be ...
-
DOSAGE AND ADMINISTRATIONIn most patients, Erythromycin Delayed-Release Tablets are well absorbed and may be dosed orally without regard to meals. However, optimal blood levels are obtained when Erythromycin ...
-
HOW SUPPLIEDErythromycin Delayed-Release Tablets, USP, are supplied as white oval enteric-coated tablets debossed on one side with the letter A, and on the other side with a two letter Code designation, EC ...
-
REFERENCESCommittee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association: Prevention of Rheumatic Fever ...
-
SPL UNCLASSIFIED SECTIONEDAG-PI-02 - Revised: April 2019 - Wilshire Pharmaceuticals, Inc. Atlanta, GA 30328 USA - (List 6304, 6320, 6321)
-
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Bottle LabelNDC52536-180-03 - 30 Tablets - ERYTHROMYCIN - DELAYED-RELEASE - TABLETS, USP - ENTERIC-COATED - 250 mg - Rx only - WILSHIRE - PHARMACEUTICALS, INC
-
PRINCIPAL DISPLAY PANEL - 333 mg Tablet Bottle LabelNDC52536-183-03 - 30 Tablets - ERYTHROMYCIN - DELAYED-RELEASE - TABLETS, USP - ENTERIC-COATED - 333 mg - Rx only - WILSHIRE - PHARMACEUTICALS, INC
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle LabelNDC52536-186-03 - 30 Tablets - ERYTHROMYCIN - DELAYED-RELEASE - TABLETS, USP - ENTERIC-COATED - 500 mg - Rx only - WILSHIRE - PHARMACEUTICALS, INC
-
INGREDIENTS AND APPEARANCEProduct Information