Label: ERYTHROMYCIN tablet, film coated
- NDC Code(s): 52536-103-03, 52536-103-13, 52536-105-03, 52536-105-13
- Packager: Wilshire Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 14, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFilm-coated Tablets - Rx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Erythromycin Tablets and other antibacterial drugs, Erythromycin Tablets should ...
-
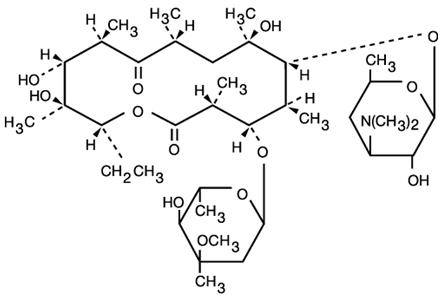
DESCRIPTIONErythromycin Tablets, USP are an antibacterial product containing erythromycin, USP, in a unique, nonenteric film coating for oral administration. Erythromycin Tablets are available in two ...
-
CLINICAL PHARMACOLOGYOrally administered erythromycin base and its salts are readily absorbed in the microbiologically active form. Interindividual variations in the absorption of erythromycin are, however, observed ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of Erythromycin Tablets, USP and other antibacterial drugs, Erythromycin Tablets, USP should be used only to ...
-
CONTRAINDICATIONSErythromycin is contraindicated in patients with known hypersensitivity to this antibiotic. Erythromycin is contraindicated in patients taking terfenadine, astemizole, cisapride, pimozide ...
-
WARNINGSHepatotoxicity - There have been reports of hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, occurring in ...
-
PRECAUTIONSGeneral - Prescribing Erythromycin Tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and ...
-
ADVERSE REACTIONSThe most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. They include nausea, vomiting, abdominal pain, diarrhea and anorexia. Symptoms of ...
-
OVERDOSAGEIn case of overdosage, erythromycin should be discontinued. Overdosage should be handled with the prompt elimination of unabsorbed drug and all other appropriate measures should be ...
-
DOSAGE AND ADMINISTRATIONIn most patients, Erythromycin Tablets are well absorbed and may be dosed orally without regard to meals. However, optimal blood levels are obtained when Erythromycin Tablets are given in the ...
-
HOW SUPPLIEDErythromycin Tablets are supplied as pink, unscored oval tablets in the following strengths and packages. 250 mg tablets (debossed with EB): Bottles of 30(NDC 52536-103-03) Bottles of ...
-
REFERENCESCommittee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association: Prevention of Rheumatic Fever ...
-
SPL UNCLASSIFIED SECTIONEBAG-PI-02 - Revised: January 2019 - Wilshire Pharmaceuticals, Inc. Atlanta, GA 30328 - (Nos. 6326 and 6227)
-
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Bottle LabelNDC 52536-103-03 - 30 Tablets - ERYTHROMYCIN - TABLETS, USP - Film-coated Tablets - 250 mg - Erythromycin, USP - Tablet identification code format - change adopted October, 2000. Rx ...
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle LabelNDC 52536-105-03 - 30 Tablets - ERYTHROMYCIN - TABLETS, USP - Film-coated Tablets - 500 mg - Erythromycin, USP - Tablet identification code format - change adopted October, 2000. Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information