Label: SUTAB- sodium sulfate, magnesium sulfate, and potassium chloride tablet
- NDC Code(s): 52268-201-01
- Packager: Braintree Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use - SUTAB - ®safely and effectively. See full prescribing information for - SUTAB. SUTAB (sodium sulfate, magnesium sulfate, and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESUTAB is indicated for the cleansing of the colon as a preparation for colonoscopy in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Preparation and Administration Instructions - Correct fluid and electrolyte abnormalities before treatment with SUTAB - [see Warnings and Precautions ( 5.1) ...
-
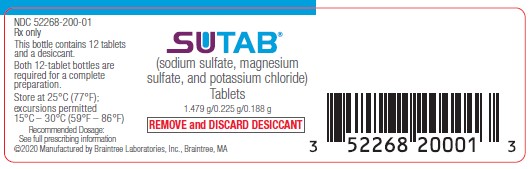
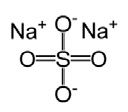
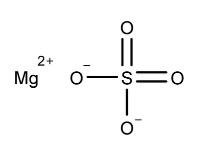
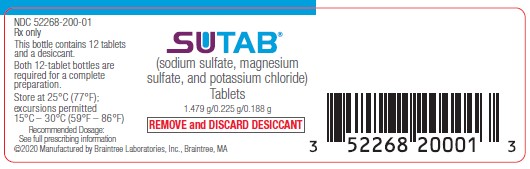
3 DOSAGE FORMS AND STRENGTHSTablets: 1.479 g sodium sulfate, 0.225 g magnesium sulfate, and 0.188 g potassium chloride. The tablets are white to off-white, film coated, oblong, and biconvex with flat sides, debossed with ...
-
4 CONTRAINDICATIONSSUTAB is contraindicated in the following conditions: Gastrointestinal obstruction or ileus - [see Warnings and Precautions ( 5.6) Bowel Perforation - [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Fluid and Electrolyte Abnormalities - Advise all patients to hydrate adequately before, during, and after the use of SUTAB. If a patient develops significant vomiting or signs of ...
-
6 ADVERSE REACTIONSThe following serious or otherwise important adverse reactions for bowel preparations are described elsewhere in the labeling: Serious Fluid and Electrolyte Abnormalities - [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Drugs That May Increase Risks of Fluid and Electrolyte Abnormalities - Use caution when prescribing SUTAB to patients taking medications that increase the risk of fluid and electrolyte ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on SUTAB use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
10 OVERDOSAGEOverdosage of more than the recommended dose of SUTAB may lead to severe electrolyte disturbances, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances - [see ...
-
11 DESCRIPTIONSUTAB (sodium sulfate, magnesium sulfate, and potassium chloride) tablets is an orally administered osmotic laxative and is provided as two bottles, each containing 12 tablets. Each tablet ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The primary mode of action is osmotic action of sodium sulfate and magnesium sulfate, which induce a laxative effect. The physiological consequence is increased water ...
-
13 NONCLINICAL TOXICOLOGY13.2 Animal Toxicology and/or Pharmacology - Animal toxicology studies with sodium sulfate, magnesium sulfate, and potassium chloride (SUTAB) have not been conducted. Sulfate salts of sodium ...
-
14 CLINICAL STUDIESThe colon cleansing efficacy of SUTAB was evaluated in two randomized, single-blind, active-controlled, multicenter trials (Study 1 and Study 2). These trials included adult subjects undergoing ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach tablet of SUTAB contains 1.479 g sodium sulfate, 0.225 g magnesium sulfate, and 0.188 g potassium chloride. The tablets are white to off-white, film coated, oblong, and biconvex with flat ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Instruct patients: Administration of two doses of SUTAB (24 tablets) are required for a ...
-
MEDICATION GUIDEMEDICATION GUIDE - SUTAB - ®(Sootab) (sodium sulfate, potassium sulfate and potassium chloride) tablets, for oral use - Read and understand these Medication Guide ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Carton Label - NDC 52268-201-01 - Please see www.sebelapharma.com for patent information. SUTAB - (sodium sulfate, magnesium sulfate, and potassium chloride) Tablets ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Bottle Label - NDC 52268-200-01 - Rx only - SUTAB - (sodium sulfate, magnesium sulfate, and potassium chloride) Tablets - 1.479g/0.225g/0.188g - REMOVE ...
-
INGREDIENTS AND APPEARANCEProduct Information