Label: TETRACYCLINE HYDROCHLORIDE capsule
- NDC Code(s): 51991-906-01, 51991-906-05, 51991-906-33, 51991-907-01, view more
- Packager: Breckenridge Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Oral Use - Rx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of tetracycline hydrochloride and other antibacterial drugs, tetracycline hydrochloride ...
-
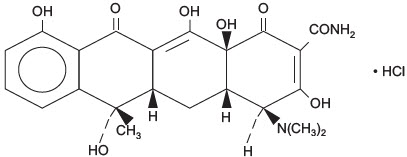
DESCRIPTIONTetracycline is a yellow crystalline powder. Tetracycline is stable in air but exposure to strong sunlight causes it to darken. Its potency is affected in solutions of pH below 2 and is rapidly ...
-
CLINICAL PHARMACOLOGYTetracyclines are readily absorbed and are bound to plasma protein in varying degrees. They are concentrated by the liver in the bile and excreted in the urine and feces at high concentrations in ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of tetracycline hydrochloride and other antibacterial drugs, tetracycline hydrochloride should be used only to ...
-
CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
WARNINGSTooth Development - The use of drugs of the tetracycline-class during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of ...
-
PRECAUTIONSGeneral - As with other antibacterials, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, discontinue antibacterial and institute ...
-
ADVERSE REACTIONSGastrointestinal: anorexia, nausea, epigastric distress, vomiting, diarrhea, glossitis, black hairy tongue, dysphagia, enterocolitis, and inflammatory lesions (with Candida overgrowth) in the ...
-
OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Tetracycline is not dialyzable.
-
DOSAGE AND ADMINISTRATIONAdults - Usual daily dose, 1 gram as 500 mg twice a day or 250 mg four times a day. Higher doses such as 500 mg four times a day may be required for severe infections or for those infections ...
-
HOW SUPPLIEDTetracycline Hydrochloride Capsules, USP are available as: 250 mg: Blue opaque cap and yellow opaque Body, size '2', cap and body imprinted B906 in black Ink. Available in bottles of: Bottle of 30 ...
-
ANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGYHyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by oxytetracycline, doxycycline, minocycline, tetracycline PO4 and ...
-
SPL UNCLASSIFIED SECTIONRx only - Manufactured by: Cohance Lifesciences Limited - Plot No. A-19/C, A-23A & A-23B, Road No.18, IDA Nacharam, Uppal Mandal, Medchal- Malkajgiri District -500 076, Telangana state ...
-
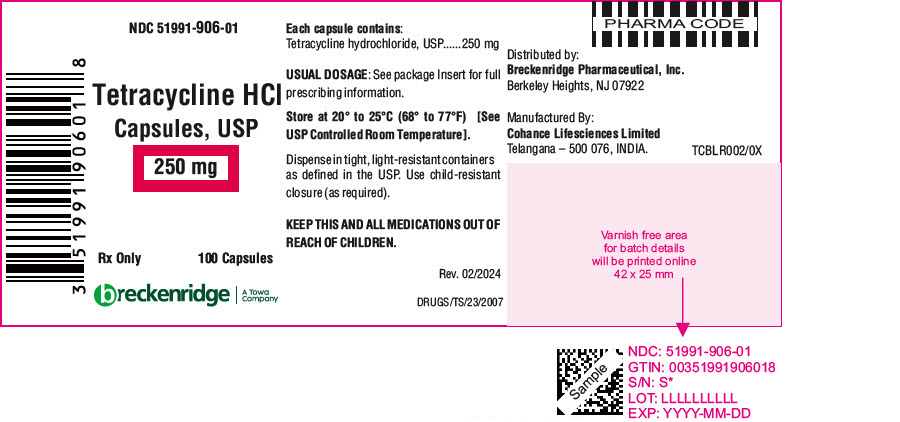
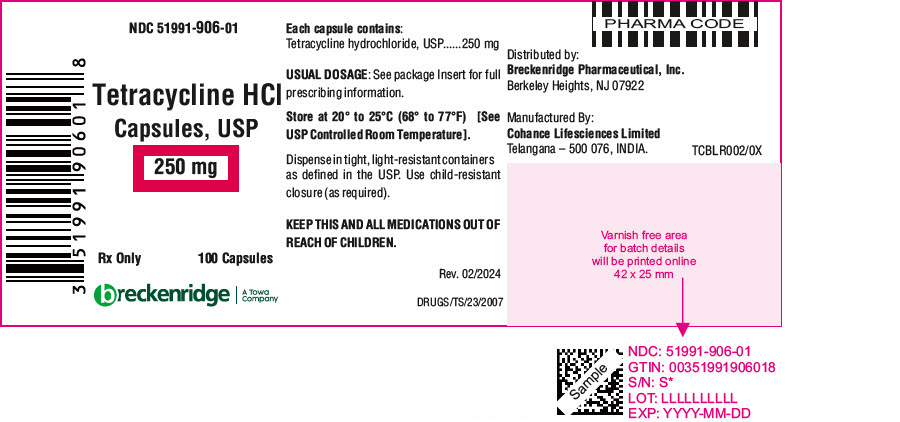
PRINCIPAL DISPLAY PANEL - 250 mg Capsule Bottle LabelNDC 51991-906-01 - Tetracycline HCl - Capsules, USP - 250 mg - Rx Only - 100 Capsules - breckenridge - A Towa - Company
-
PRINCIPAL DISPLAY PANEL - 500 mg Capsule Bottle LabelNDC 51991-907-01 - Tetracycline HCl - Capsules, USP - 500 mg - Rx Only - 100 Capsules - breckenridge - A Towa - Company
-
INGREDIENTS AND APPEARANCEProduct Information