Label: PROPRANOLOL HYDROCHLORIDE capsule, extended release
- NDC Code(s): 51991-817-01, 51991-817-05, 51991-818-01, 51991-818-05, view more
- Packager: Breckenridge Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
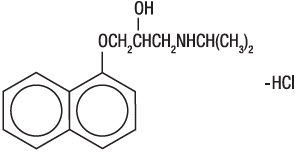
DESCRIPTIONPropranolol hydrochloride is a synthetic beta-adrenergic receptor-blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,(±)-. It's ...
-
CLINICAL PHARMACOLOGYGeneral - Propranolol is a nonselective, beta-adrenergic receptor-blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic ...
-
INDICATIONS AND USAGEHypertension - Propranolol Hydrochloride Extended-Release Capsules, USP, are indicated in the management of hypertension. They may be used alone or used in combination with other antihypertensive ...
-
CONTRAINDICATIONSPropranolol is contraindicated in 1) cardiogenic shock; 2) sinus bradycardia and greater than first-degree block; 3) bronchial asthma; and 4) in patients with known hypersensitivity to propranolol ...
-
WARNINGSAngina Pectoris - There have been reports of exacerbation of angina and, in some cases, myocardial infarction, following abrupt discontinuance of propranolol therapy. Therefore, when ...
-
PRECAUTIONSGeneral - Propranolol should be used with caution in patients with impaired hepatic or renal function. Propranolol Hydrochloride Extended-Release Capsules, USP, are not indicated for the ...
-
ADVERSE REACTIONSThe following adverse events were observed and have been reported in patients using propranolol. Cardiovascular - Bradycardia; congestive heart failure; intensification of AV block; hypotension ...
-
OVERDOSAGEPropranolol is not significantly dialyzable. In the event of overdosage or exaggerated response, the following measures should be employed: General - If ingestion is or may have been recent ...
-
DOSAGE AND ADMINISTRATIONGeneral - Propranolol Hydrochloride Extended-Release Capsules, USP, provide propranolol hydrochloride in a sustained-release capsule for administration once daily. If patients are switched from ...
-
HOW SUPPLIEDPropranolol Hydrochloride Extended-Release Capsules, USP. Each white/opaque capsule, imprinted with "60"on cap and "RD203" on body contains 60 mg of propranolol hydrochloride in bottles of 100 ...
-
SPL UNCLASSIFIED SECTIONPropranolol Hydrochloride Extended-Release Capsules, USP, a DIFFUCAPS® drug delivery product, manufactured by Adare Pharmaceuticals, Inc. You can also ask your doctor or pharmacist for information ...
-
PRINCIPAL DISPLAY PANEL - 60 mg Capsule Bottle LabelNDC 51991-817-01 - Propranolol - Hydrochloride - Extended-Release - Capsules, USP - 60 mg - breckenridge - A Towa - Company - Rx Only - 100 Capsules
-
PRINCIPAL DISPLAY PANEL - 80 mg Capsule Bottle LabelNDC 51991-818-01 - Propranolol - Hydrochloride - Extended-Release - Capsules, USP - 80 mg - breckenridge - A Towa - Company - Rx Only - 100 Capsules
-
PRINCIPAL DISPLAY PANEL - 120 mg Capsule Bottle LabelNDC 51991-819-01 - Propranolol - Hydrochloride - Extended-Release - Capsules, USP - 120 mg - breckenridge - A Towa - Company - Rx Only - 100 Capsules
-
PRINCIPAL DISPLAY PANEL - 160 mg Capsule Bottle LabelNDC 51991-820-01 - Propranolol - Hydrochloride - Extended-Release - Capsules, USP - 160 mg - breckenridge - A Towa - Company - Rx Only - 100 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information