Label: RIZATRIPTAN BENZOATE tablet, orally disintegrating
- NDC Code(s): 51991-362-78, 51991-362-99, 51991-363-78, 51991-363-99
- Packager: Breckenridge Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RIZATRIPTAN BENZOATE ORALLY DISINTEGRATING TABLETS safely and effectively. See full prescribing information for RIZATRIPTAN BENZOATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERizatriptan benzoate orally disintegrating tablets are indicated for the acute treatment of migraine with or without aura in adults and in pediatric patients 6 to 17 years old. Limitations of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information in Adults - The recommended starting dose of rizatriptan benzoate orally disintegrating tablets is either 5 mg or 10 mg for the acute treatment of migraines in adults. The ...
-
3 DOSAGE FORMS AND STRENGTHSRizatriptan Benzoate Orally Disintegrating Tablets - The 5 mg tablets are white to off-white, round, uncoated tablets, debossed "5" on one side and plain on the other side. The 10 mg tablets ...
-
4 CONTRAINDICATIONSRizatriptan benzoate orally disintegrating tablets are contraindicated in patients with: Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina - Rizatriptan benzoate should not be given to patients with ischemic or vasospastic coronary artery disease. There have ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Propranolol - The dose of rizatriptan benzoate should be adjusted in propranolol-treated patients, as propranolol has been shown to increase the plasma AUC of rizatriptan by 70% [see Dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available human data on the use of rizatriptan benzoate orally disintegrating tablets in pregnant women are not sufficient to draw conclusions about ...
-
10 OVERDOSAGENo overdoses of rizatriptan benzoate were reported during clinical trials in adults. Some adult patients who received 40 mg of rizatriptan benzoate either a single dose or as two doses with a ...
-
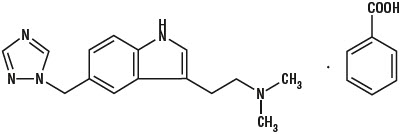
11 DESCRIPTIONRizatriptan benzoate orally disintegrating tablets contain rizatriptan benzoate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist. Rizatriptan benzoate is described chemically as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Rizatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Rizatriptan benzoate presumably exerts its therapeutic effects in the treatment of migraine ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Oral carcinogenicity studies of rizatriptan were conducted in mice (100 weeks) and rats (106 weeks) at doses of up ...
-
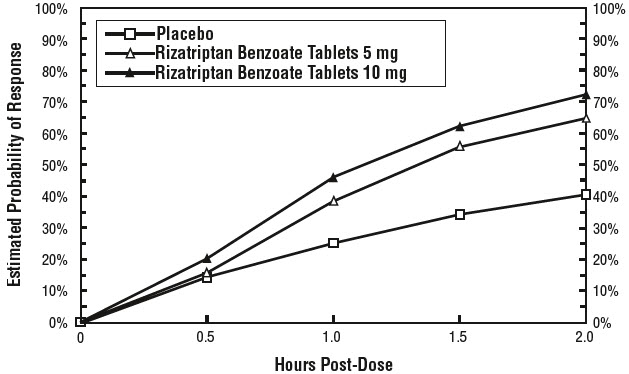
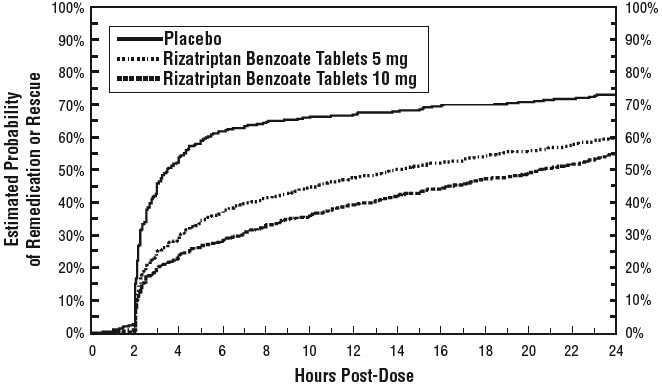
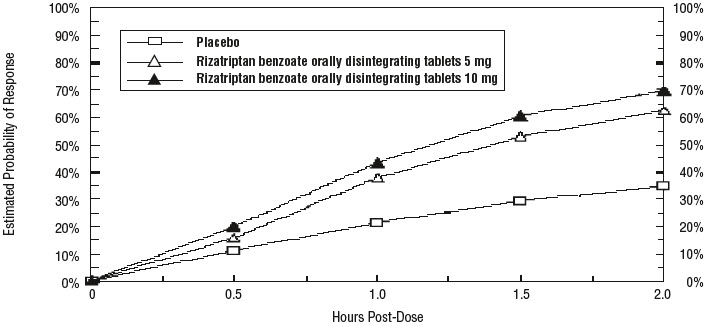
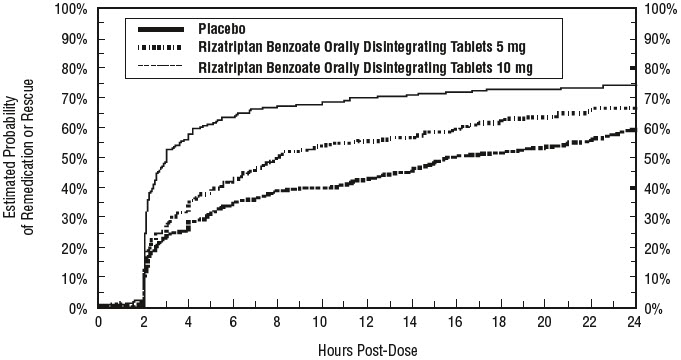
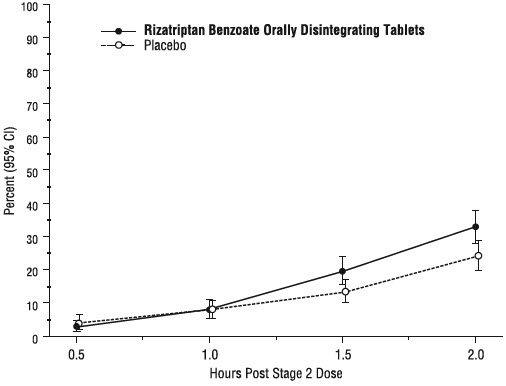
14 CLINICAL STUDIES14.1 Adults - The efficacy of rizatriptan benzoate tablets was established in four multicenter, randomized, placebo-controlled trials. Patients enrolled in these studies were primarily female ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRizatriptan Benzoate Orally Disintegrating Tablets are available in strengths of 5 mg and 10 mg containing 7.265 mg or 14.53 mg of rizatriptan benzoate, USP equivalent to 5 mg or 10 mg of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-Approved Patient Labeling (Patient Information). Risk of Myocardial Ischemia and/or Infarction, Prinzmetal's Angina, Other Vasospasm-related Events, and ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Natco Pharma Limited, Kothur- 509228, India. Distributed by: Breckenridge Pharmaceutical, Inc. Berkeley Heights, NJ 07922 - Rev.: Jan/2024 - xxxxxx
-
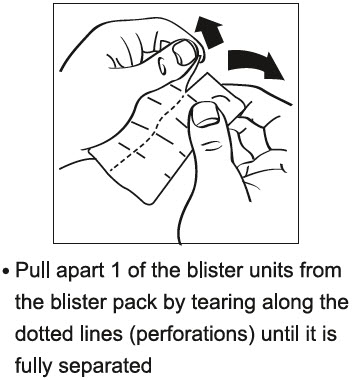
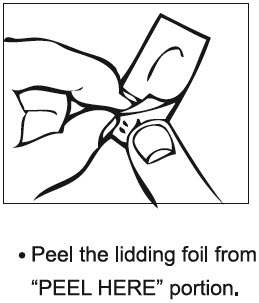
PATIENT INFORMATIONRIZATRIPTAN BENZOATE (rye" za trip' tan ben' zoe ate) ORALLY DISINTEGRATING TABLETS - 5 mg and 10 mg - Read this Patient Information before you start taking rizatriptan benzoate and each time you ...
-
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack CartonNDC 51991-362-78 - Rizatriptan Benzoate Orally - Disintegrating Tablets - 5 mg* PHARMACIST: Dispense the accompanying Patient Information Leaflet to each patient. APPLY PHARMACY LABEL HERE - breckenridge ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Blister Pack CartonNDC 51991-363-78 - Rizatriptan Benzoate Orally - Disintegrating Tablets - 10 mg* PHARMACIST: Dispense the accompanying Patient Information Leaflet to each patient. APPLY PHARMACY LABEL ...
-
INGREDIENTS AND APPEARANCEProduct Information