Label: SILDENAFIL- sildenafil citrate powder, for suspension
- NDC Code(s): 51672-4231-8
- Packager: Taro Pharmaceuticals U.S.A. Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SILDENAFIL FOR ORAL SUSPENSION safely and effectively. See full prescribing information for SILDENAFIL FOR ORAL SUSPENSION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAdults - Sildenafil for Oral Suspension is indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage in Adults - Oral Dosage - The recommended dosage of Sildenafil for Oral Suspension is 20 mg three times a day - [see - Clinical Studies (14)] ...
-
3 DOSAGE FORMS AND STRENGTHSSildenafil for Oral Suspension - White to off-white powders containing 1.57 g of sildenafil citrate (equivalent to 1.12 g of sildenafil) in a bottle for reconstitution to 10 mg/mL. Following ...
-
4 CONTRAINDICATIONSSildenafil for Oral Suspension is contraindicated in patients with: Concomitant use of organic nitrates in any form, either regularly or intermittently, because of the greater risk of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Sildenafil has vasodilatory properties, resulting in mild and transient decreases in blood pressure. Before prescribing Sildenafil for Oral Suspension, carefully consider ...
-
6 ADVERSE REACTIONSThe following serious adverse events are discussed elsewhere in the labeling: Hypotension - [see - Warnings and Precautions (5.1)] Vision Loss - [see - Warnings and ...
-
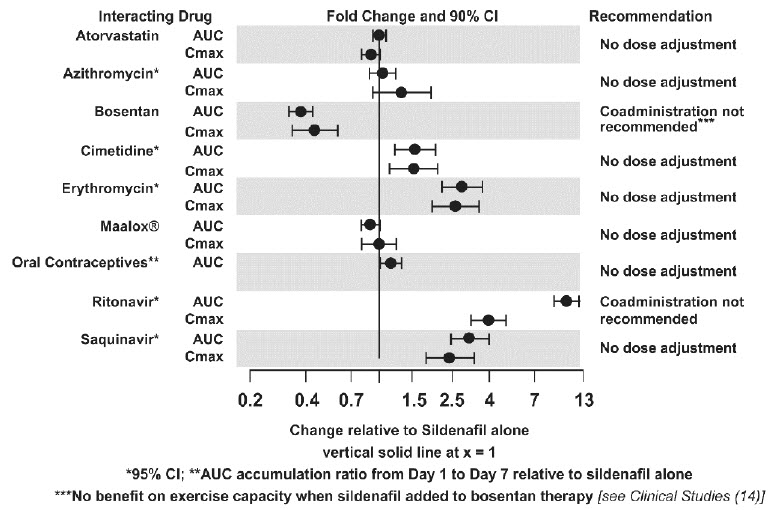
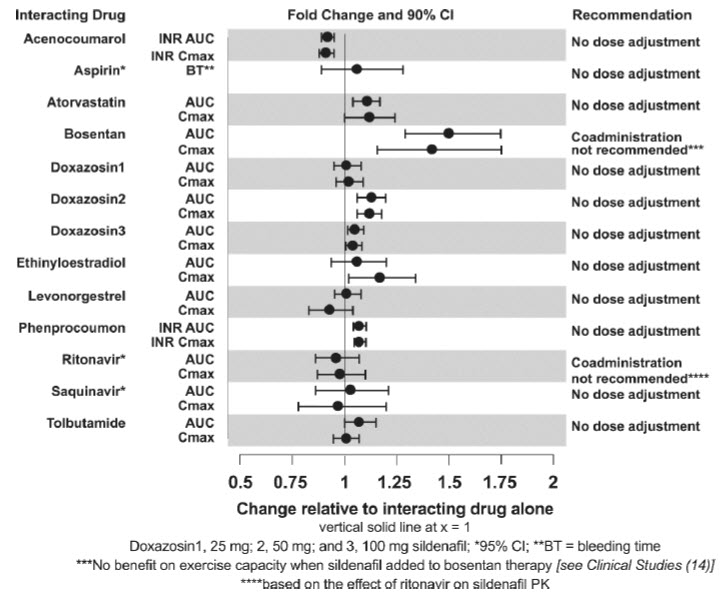
7 DRUG INTERACTIONSNitrates - Concomitant use of Sildenafil for Oral Suspension with nitrates in any form is contraindicated - [see - Contraindications (4)] . Strong CYP3A ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited published data from randomized controlled trials, case-controlled trials, and case series do not report a clear association with sildenafil and major ...
-
10 OVERDOSAGEIn studies with healthy volunteers of single doses up to 800 mg, adverse events were similar to those seen at lower doses but rates and severities were increased. In cases of overdose, standard ...
-
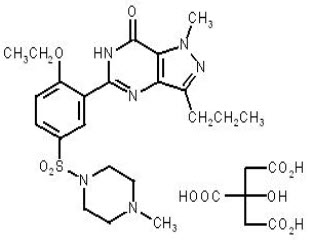
11 DESCRIPTIONSildenafil for Oral Suspension, a phosphodiesterase-5 (PDE-5) inhibitor, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sildenafil is an inhibitor of cGMP specific PDE-5 in the smooth muscle of the pulmonary vasculature, where PDE-5 is responsible for degradation of cGMP. Sildenafil ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Sildenafil was not carcinogenic when administered to rats for up to 24 months at 60 mg/kg/day, a dose resulting in total systemic ...
-
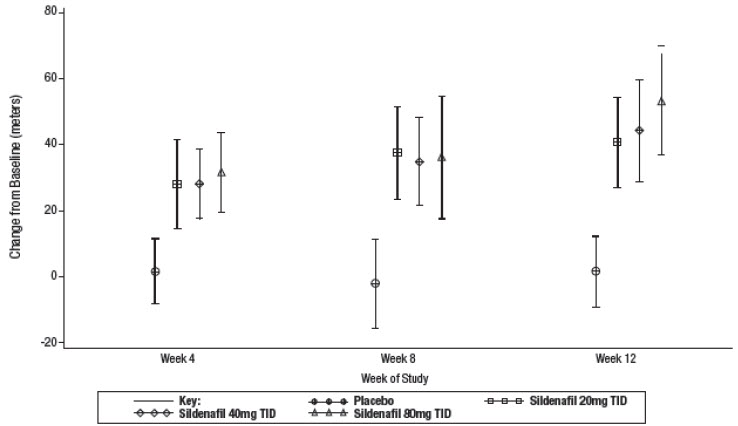
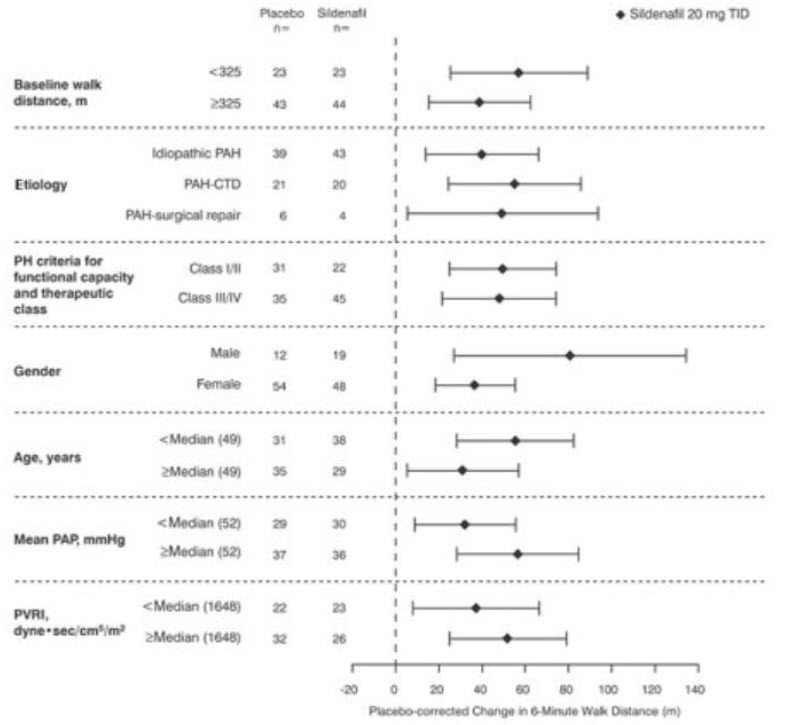
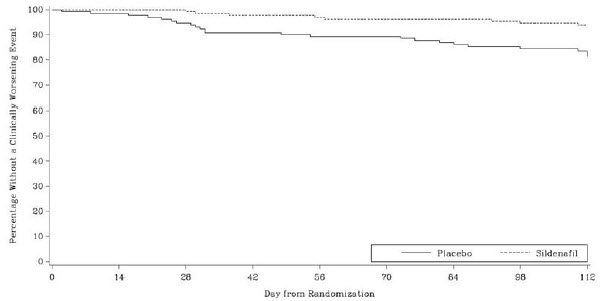
14 CLINICAL STUDIESSUPER-1 (NCT00644605) - Sildenafil for Oral Suspension monotherapy [20 mg, 40 mg, and 80 mg three times a day] A randomized, double-blind, placebo-controlled study of Sildenafil for Oral ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSildenafil powder for oral suspension is supplied in amber glass bottles. Each bottle contains white to off-white powders containing 1.57 g of sildenafil citrate (equivalent to 1.12 g sildenafil) ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Inform patients of contraindication of Sildenafil for Oral Suspension with regular ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. PATIENT INFORMATION - Sildenafil (sil den⸍ a fil) for Oral Suspension - What is the ...
-
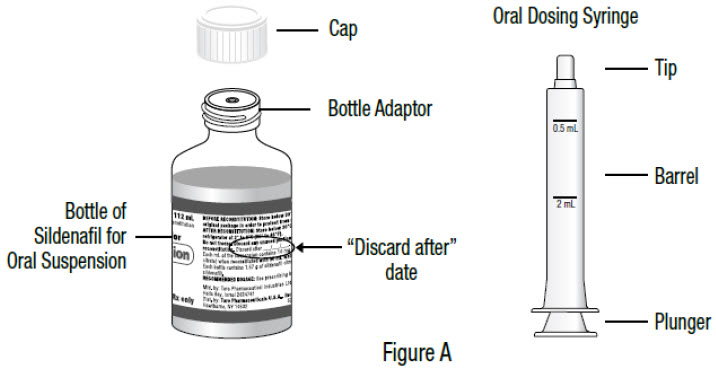
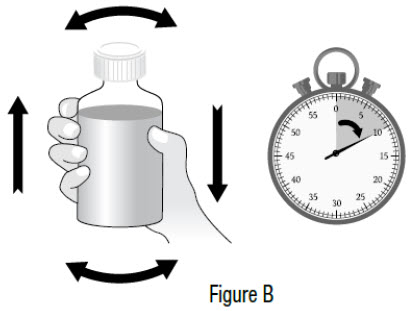
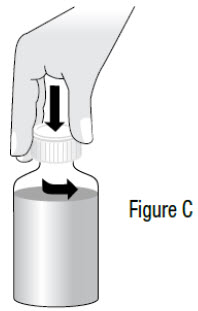
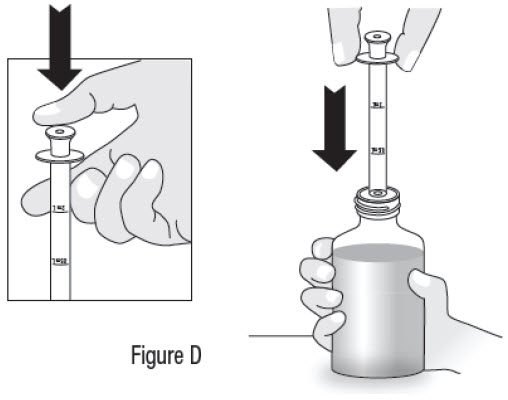
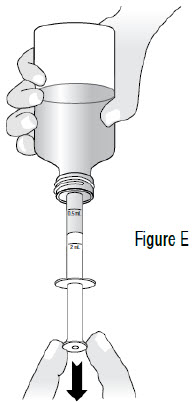
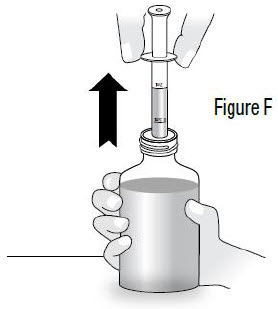
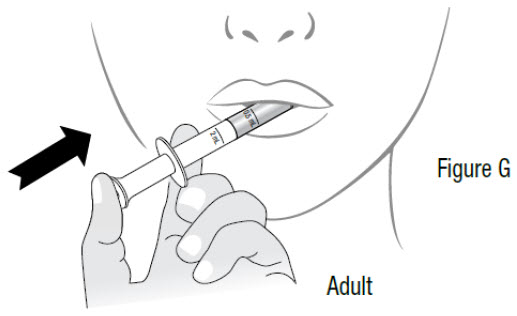
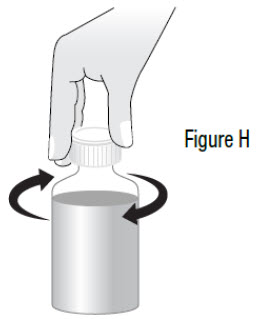
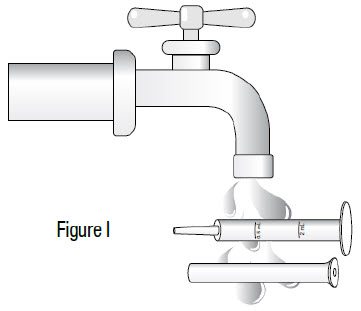
INSTRUCTIONS FOR USEInstructions for Use - Sildenafil (sil den⸍ a fil) Oral Suspension - Read this Instructions for Use before you start taking sildenafil oral suspension and each time you get a refill ...
-
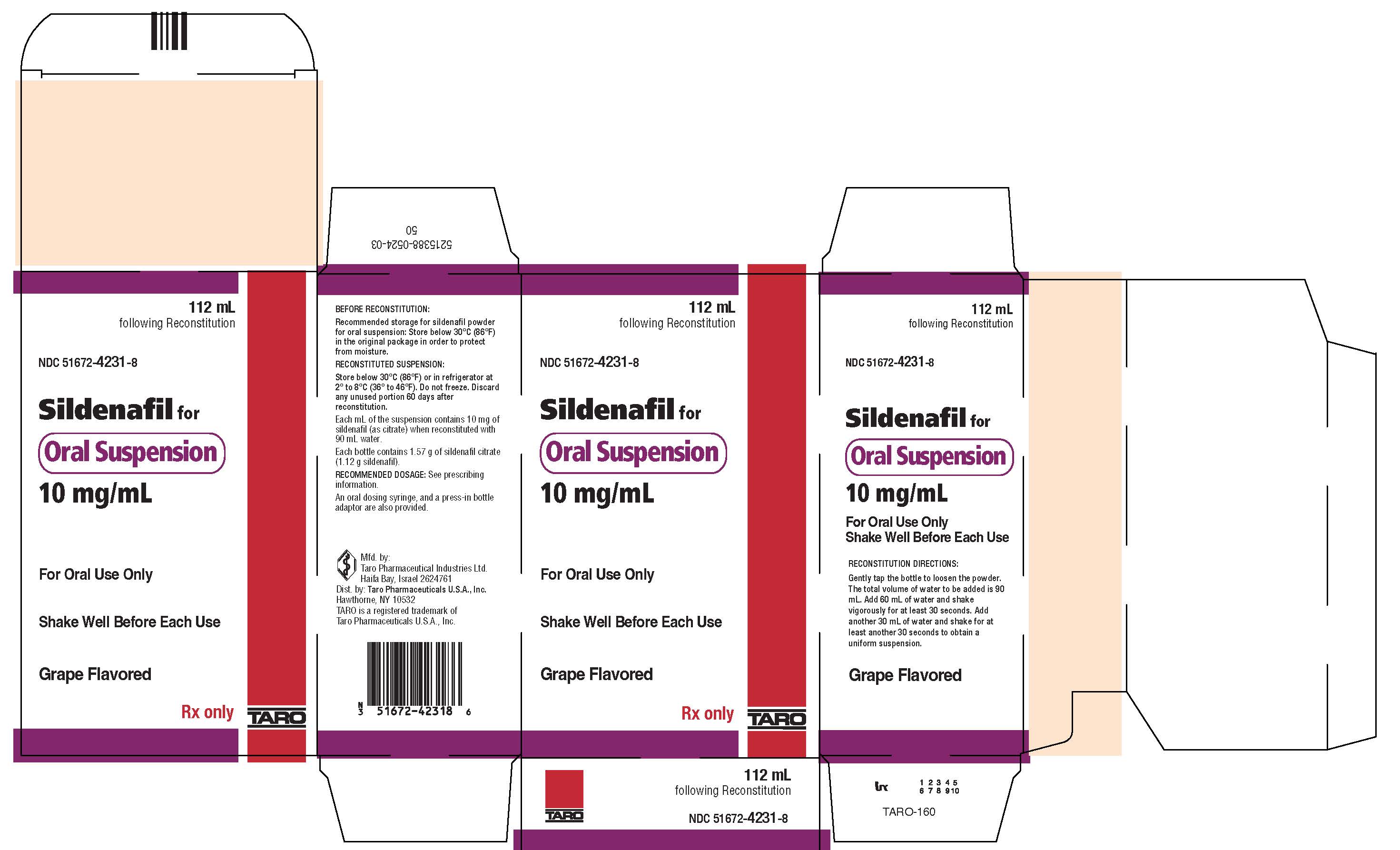
PRINCIPAL DISPLAY PANEL - 112 mL Bottle Carton112 mL - following Reconstitution - NDC 51672-4231-8 - Sildenafil for - Oral Suspension - 10 mg/mL - For Oral Use Only - Shake Well Before Each Use - Grape Flavored - Rx only - TARO
-
INGREDIENTS AND APPEARANCEProduct Information