Label: DOXEPIN HYDROCHLORIDE capsule
- NDC Code(s): 51672-4217-1, 51672-4218-1, 51672-4219-1, 51672-4220-1, view more
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 6, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of doxepin or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Doxepin is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients and PRECAUTIONS: Pediatric Use.)
Close -
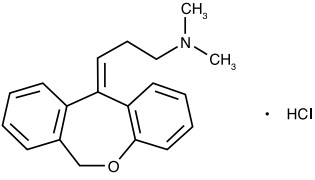
DESCRIPTIONDoxepin hydrochloride is one of a class of psychotherapeutic agents known as dibenzoxepin tricyclic compounds. The molecular formula of the compound is C19H21NO ∙ HCl having a molecular weight of ...
-
CLINICAL PHARMACOLOGYThe mechanism of action of doxepin is not definitely known. It is not a central nervous system stimulant nor a monoamine oxidase inhibitor. The current hypothesis is that the clinical effects are ...
-
INDICATIONS AND USAGEDoxepin Hydrochloride Capsules, USP are recommended for the treatment of: Psychoneurotic patients with depression and/or anxiety. Depression and/or anxiety associated with alcoholism (not to be ...
-
CONTRAINDICATIONSDoxepin hydrochloride capsules are contraindicated in individuals who have shown hypersensitivity to the drug. Possibility of cross sensitivity with other dibenzoxepines should be kept in ...
-
WARNINGSClinical Worsening and Suicide Risk - Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ...
-
PRECAUTIONSInformation for Patients - Prescribers or other health professionals should inform patients, their families and their caregivers about the benefits and risks associated with treatment with ...
-
ADVERSE REACTIONSNOTE: Some of the adverse reactions noted below have not been specifically reported with doxepin use. However, due to the close pharmacological similarities among the tricyclics, the reactions ...
-
OVERDOSAGEDeaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and ...
-
DOSAGE AND ADMINISTRATIONFor most patients with illness of mild to moderate severity, a starting daily dose of 75 mg is recommended. Dosage may subsequently be increased or decreased at appropriate intervals and according ...
-
HOW SUPPLIEDDoxepin Hydrochloride Capsules, USP are available containing doxepin hydrochloride, USP equivalent to 10 mg, 25 mg, 50 mg, 75 mg or 100 mg of doxepin. The 10 mg capsule is a hard-shell, gelatin ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 2624761 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Dispense with Medication Guide available at ...
-
MEDICATION GUIDEDispense with Medication Guide available at: https://www.taro.com/usa-medication-guides - Medication Guide - Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal ...
-
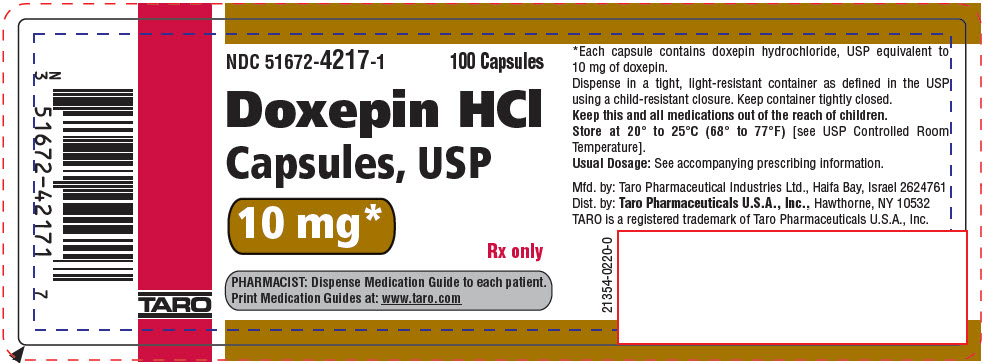
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle LabelNDC 51672-4217-1 - 100 Capsules - Doxepin HCl - Capsules, USP - 10 mg* Rx only - PHARMACIST: Dispense Medication Guide to each patient. Print Medication Guides at: www.taro.com - TARO
-
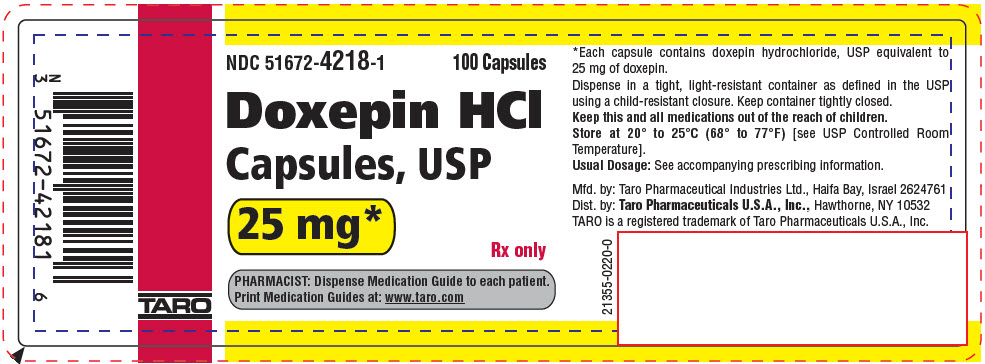
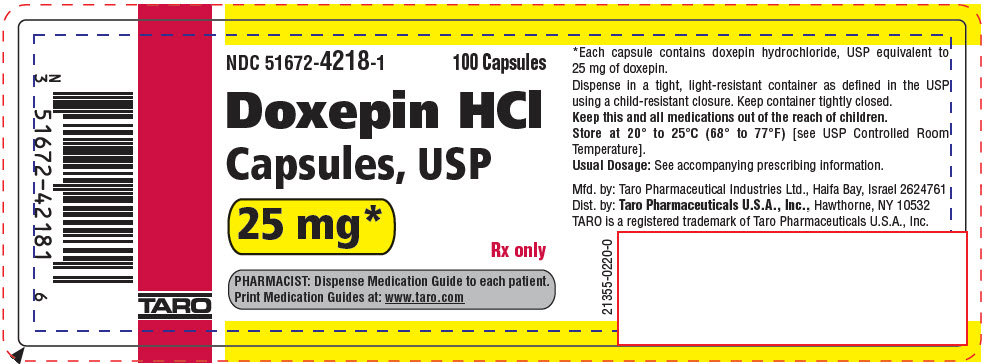
PRINCIPAL DISPLAY PANEL - 25 mg Capsule Bottle LabelNDC 51672-4218-1 - 100 Capsules - Doxepin HCl - Capsules, USP - 25 mg* Rx only - PHARMACIST: Dispense Medication Guide to each patient. Print Medication Guides at: www.taro.com - TARO
-
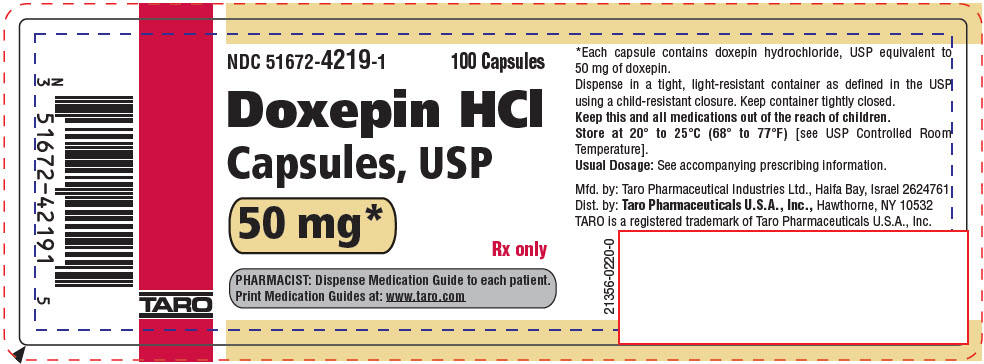
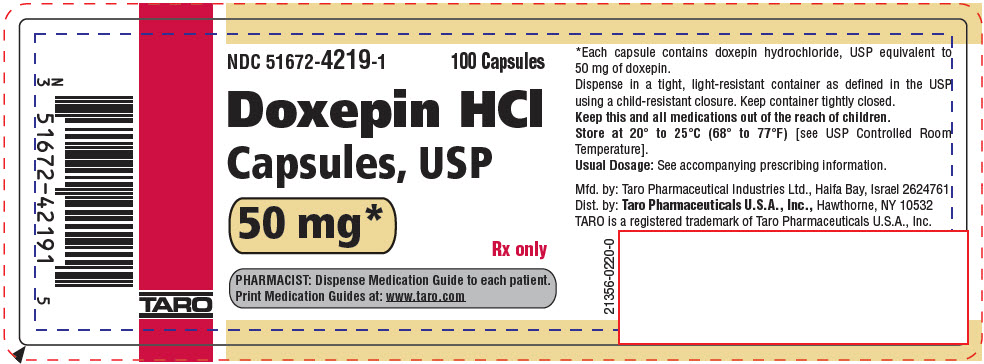
PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle LabelNDC 51672-4219-1 - 100 Capsules - Doxepin HCl - Capsules, USP - 50 mg* Rx only - PHARMACIST: Dispense Medication Guide to each patient. Print Medication Guides at: www.taro.com - TARO
-
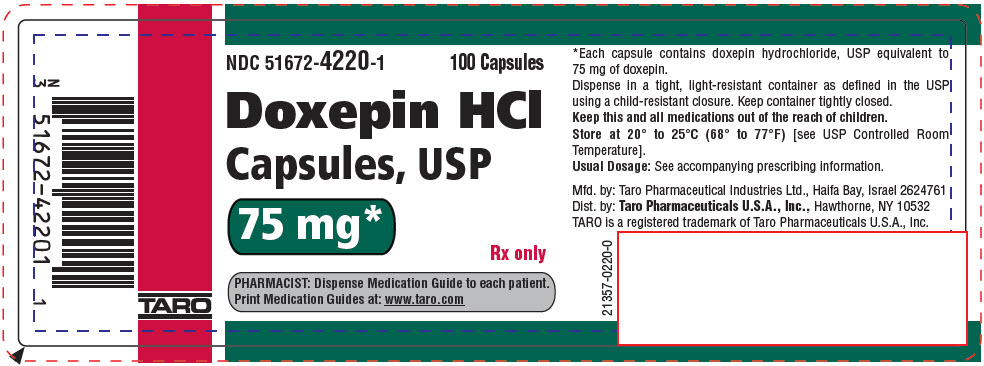
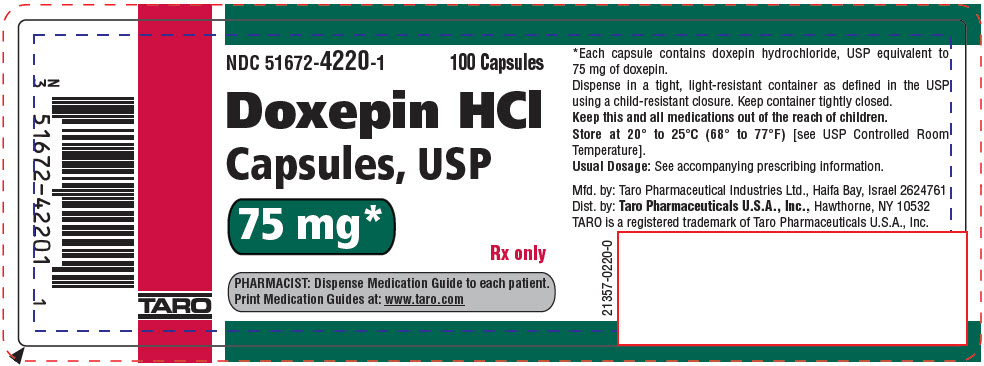
PRINCIPAL DISPLAY PANEL - 75 mg Capsule Bottle LabelNDC 51672-4220-1 - 100 Capsules - Doxepin HCl - Capsules, USP - 75 mg* Rx only - PHARMACIST: Dispense Medication Guide to each patient. Print Medication Guides at: www.taro.com - TARO
-
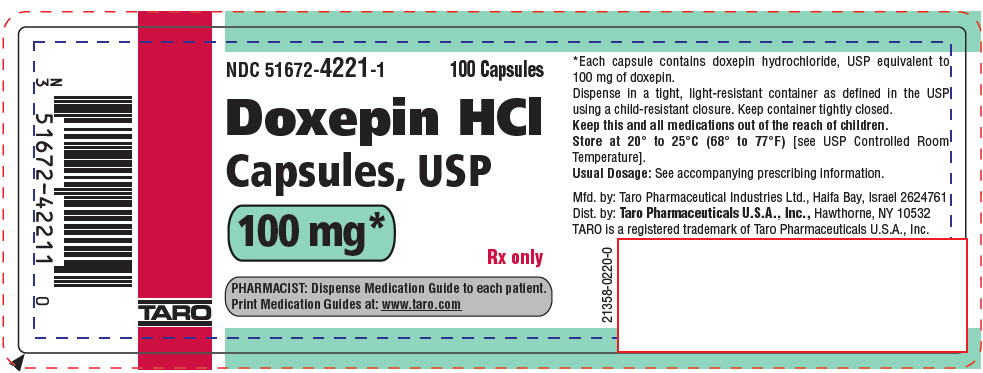
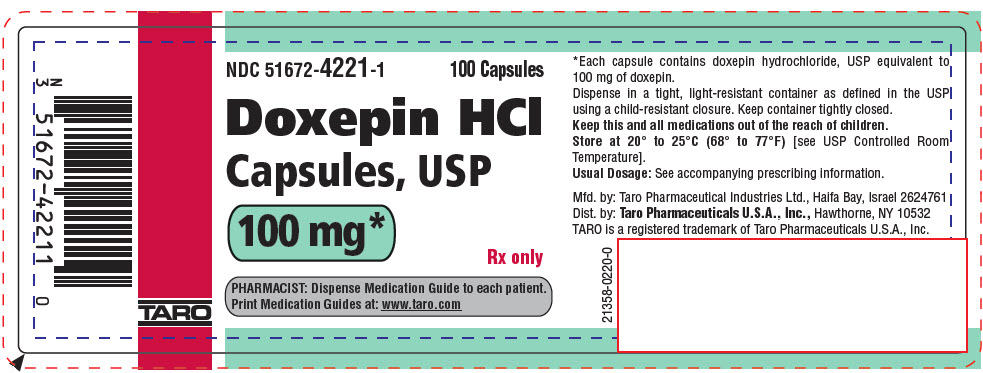
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle LabelNDC 51672-4221-1 - 100 Capsules - Doxepin HCl - Capsules, USP - 100 mg* Rx only - PHARMACIST: Dispense Medication Guide to each patient. Print Medication Guides at: www.taro.com - TARO
-
INGREDIENTS AND APPEARANCEProduct Information