Label: NYSTATIN suspension
- NDC Code(s): 51672-4117-0, 51672-4117-4, 51672-4117-9
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
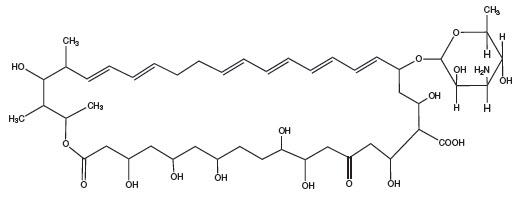
DESCRIPTIONNystatin is an antimycotic polyene antibiotic obtained from - Streptomyces noursei. Structural formula: Nystatin Oral Suspension, for oral administration, is cherry/mint flavored ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving ...
-
INDICATIONS AND USAGENystatin Oral Suspension is indicated for the treatment of candidiasis in the oral cavity.
-
CONTRAINDICATIONSThe preparation is contraindicated in patients with a history of hypersensitivity to any of its components.
-
PRECAUTIONSGeneral - This medication is not to be used for the treatment of systemic mycoses. Discontinue treatment if sensitization or irritation is reported during use. Carcinogenesis, Mutagenesis ...
-
ADVERSE REACTIONSNystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported. (See - PRECAUTIONS: General). Gastrointestinal:Diarrhea (including one case of ...
-
OVERDOSAGEOral doses of nystatin in excess of five million units daily have caused nausea and gastrointestinal upset. There have been no reports of serious toxic effects of superinfections (See - CLINICAL ...
-
DOSAGE AND ADMINISTRATIONINFANTS - 2 mL (200,000 units) four times daily (in infants and young children, use dropper to place one-half of dose in each side of mouth and avoid feeding for 5 to 10 minutes). NOTE:Limited ...
-
HOW SUPPLIEDNystatin Oral Suspension, USP, 100,000 USP Nystatin U/mL, is available as a cherry-mint flavored, light creamy yellow, ready-to-use suspension in: 2 fl oz (60 mL) bottles with 0.5 mL, 1 mL, 1.5 ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Taro Pharmaceutical Industries Ltd. Haifa Bay, Israel 2624761 - Distributed by: Taro Pharmaceuticals U.S.A., Inc. Hawthorne, N.Y. 10532 - Revised: May 2019 ...
-
PRINCIPAL DISPLAY PANEL - 60 mL Bottle LabelNDC 51672-4117-4 - 2 fl oz - (60 mL) Nystatin - Oral Suspension USP, 100,000 units/mL - SHAKE WELL BEFORE USING - CHERRY/MINT FLAVORED - TARO - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information