Label: FLUOROURACIL solution
- NDC Code(s): 51672-4062-1, 51672-4063-1
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - FOR TOPICAL USE ONLY – NOT FOR OPHTHALMIC, ORAL OR INTRAVAGINAL USE
-
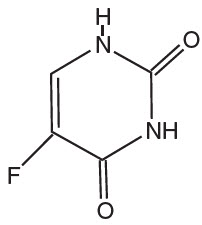
DESCRIPTIONFluorouracil Topical Solutions are topical preparations containing the fluorinated pyrimidine 5-fluorouracil, an antineoplastic antimetabolite. Fluorouracil Topical Solution consists of 2% or 5 ...
-
CLINICAL PHARMACOLOGYThere is evidence that the metabolism of fluorouracil in the anabolic pathway blocks the methylation reaction of deoxyuridylic acid to thymidylic acid. In this manner, fluorouracil interferes with ...
-
INDICATIONS AND USAGEFluorouracil is recommended for the topical treatment of multiple actinic or solar keratoses. In the 5% strength, it is also useful in the treatment of superficial basal cell carcinomas when ...
-
CONTRAINDICATIONSFluorouracil may cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies in pregnant women with either the topical or the parenteral forms of ...
-
WARNINGSApplication to mucous membranes should be avoided due to the possibility of local inflammation and ulceration. Additionally, cases of miscarriage and a birth defect (ventricular septal defect ...
-
PRECAUTIONSGeneral - There is a possibility of increased absorption through ulcerated or inflamed skin. Information for Patients - Patients should be forewarned that the reaction in the treated areas may ...
-
ADVERSE REACTIONSThe most frequent adverse reactions to fluorouracil occur locally and are often related to an extension of the pharmacological activity of the drug. These include burning, crusting, allergic ...
-
OVERDOSAGEThere have been no reports of overdosage with fluorouracil. The oral LD50 for the 5% topical cream was 234 mg/kg in rats and 39 mg/kg in dogs. These doses represented 11.7 and 1.95 mg/kg of ...
-
DOSAGE AND ADMINISTRATIONWhen fluorouracil is applied to a lesion, a response occurs with the following sequence: erythema, usually followed by vesiculation, desquamation, erosion, and re-epithelialization. Fluorouracil ...
-
HOW SUPPLIEDFluorouracil Topical Solution, USP is available in 10-mL drop dispensers containing either 2% (NDC 51672-4062-1) or 5% (NDC 51672-4063-1) fluorouracil on a weight/weight basis compounded with ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 2624761 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: September, 2022 - 5201127-0922-07 - 307
-
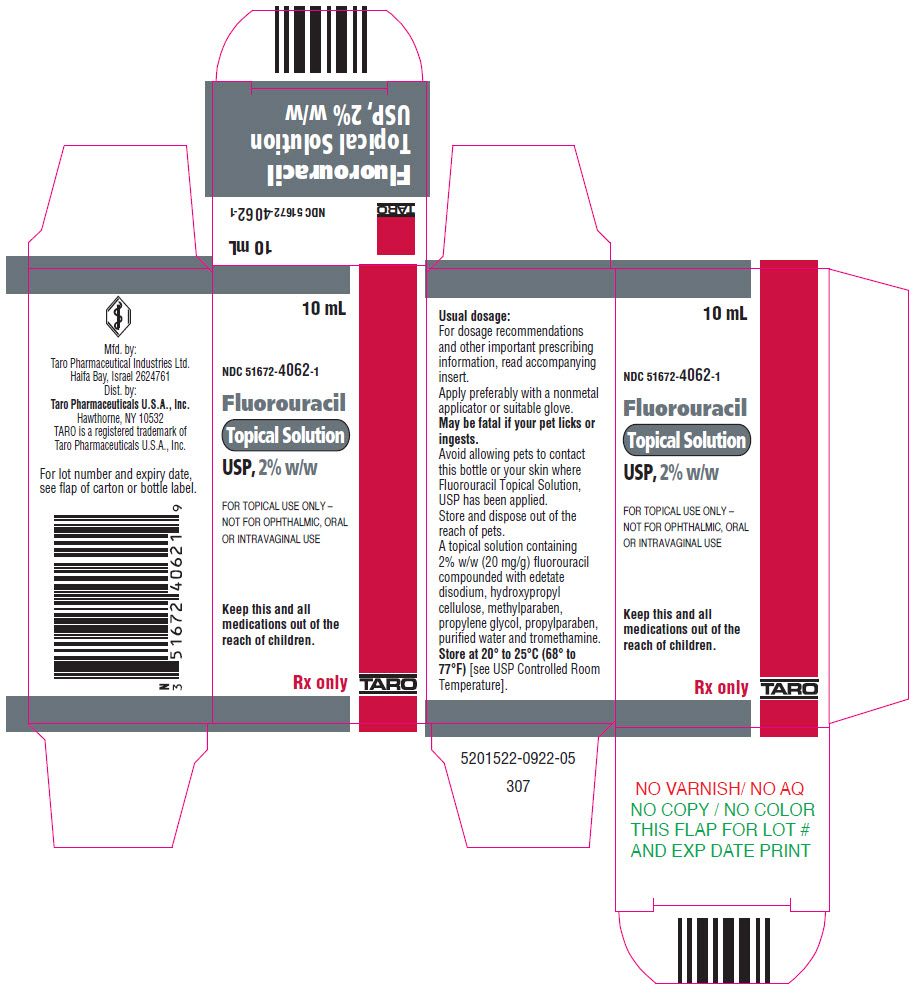
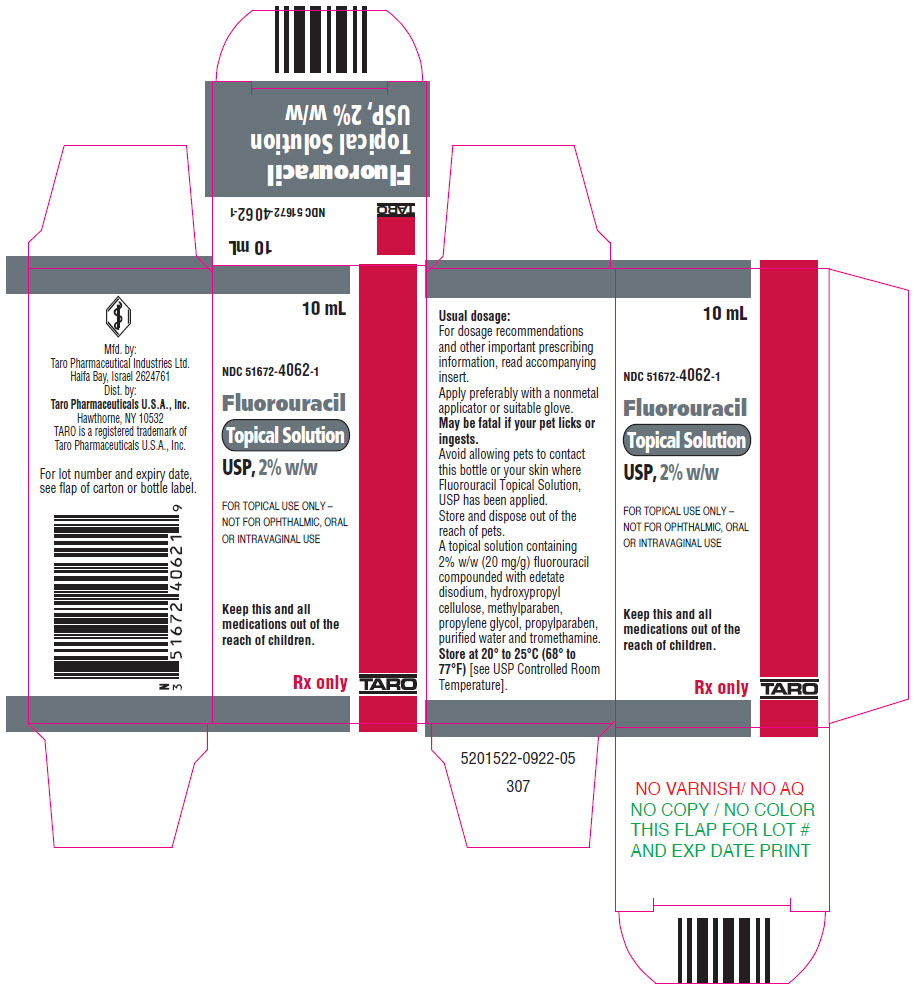
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton - 2%10 mL - NDC 51672-4062-1 - Fluorouracil - Topical Solution - USP, 2% w/w - FOR TOPICAL USE ONLY – NOT FOR OPHTHALMIC, ORAL - OR INTRAVAGINAL USE - Keep this and all - medications out of the - reach of children. Rx ...
-
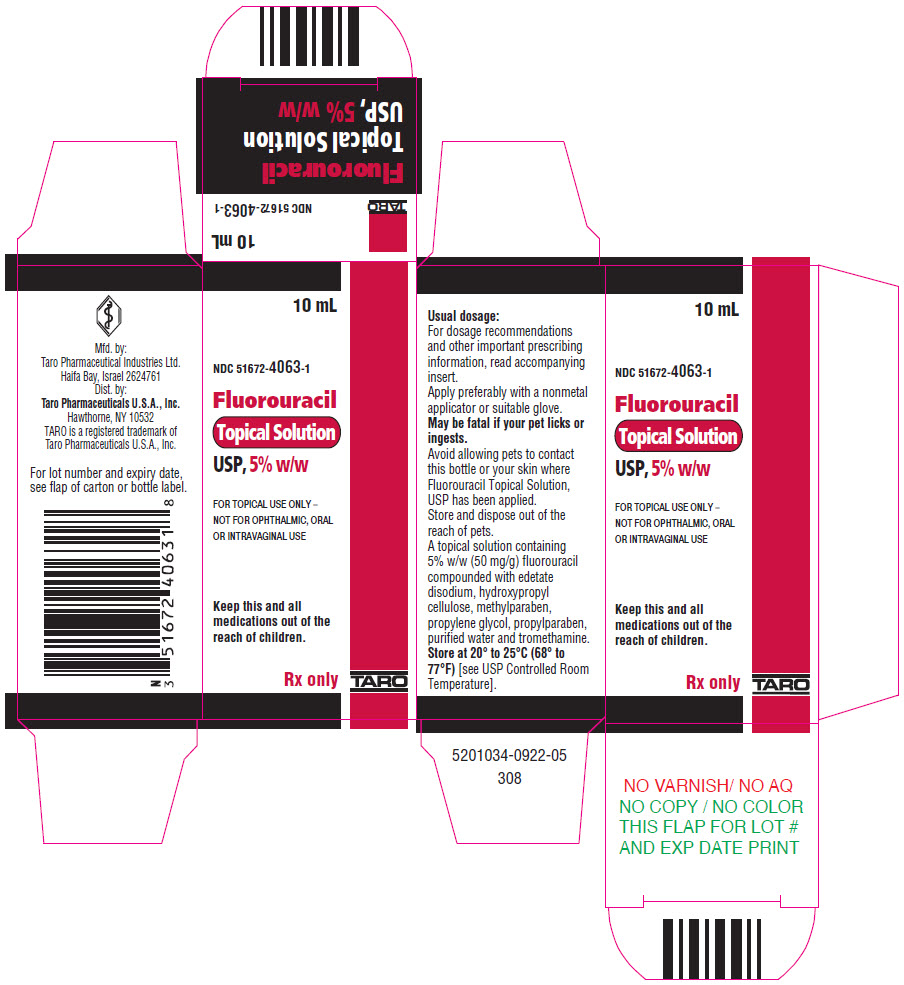
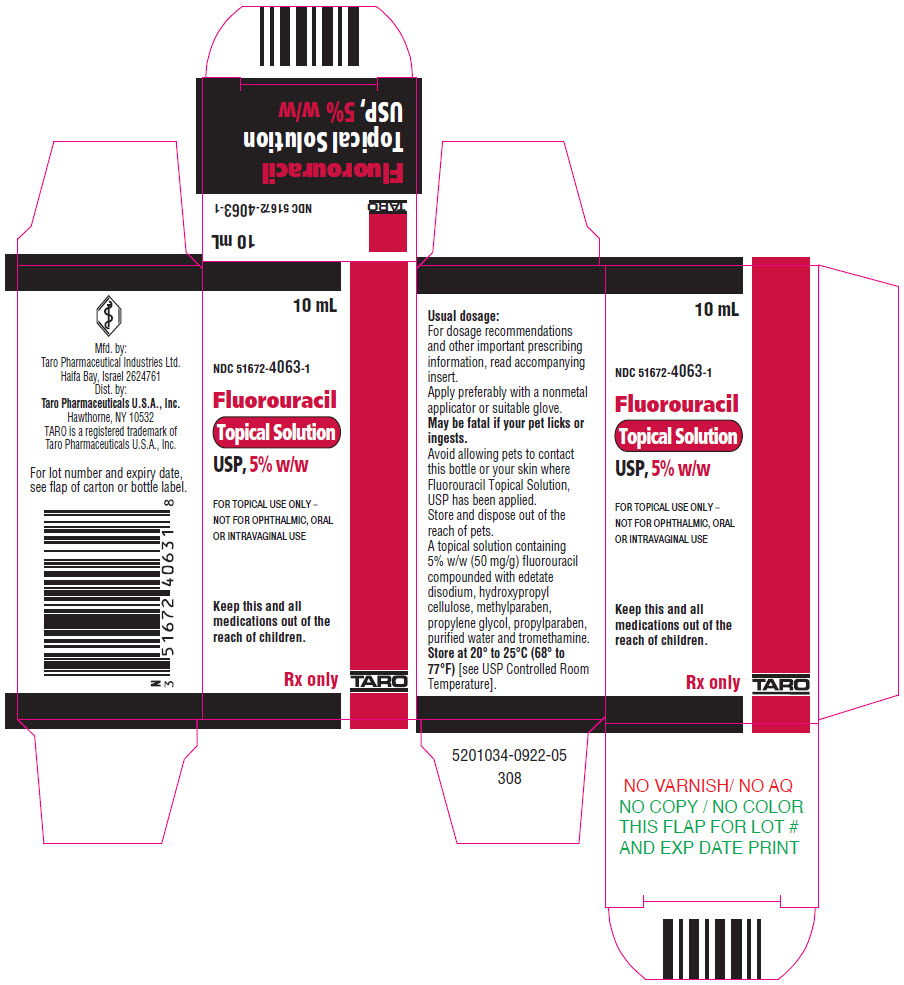
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton - 5%10 mL - NDC 51672-4063-1 - Fluorouracil - Topical Solution - USP, 5% w/w - FOR TOPICAL USE ONLY – NOT FOR OPHTHALMIC, ORAL - OR INTRAVAGINAL USE - Keep this and all - medications out of the - reach of children. Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information