Label: ACETAZOLAMIDE tablet
- NDC Code(s): 51672-4022-1, 51672-4023-1
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
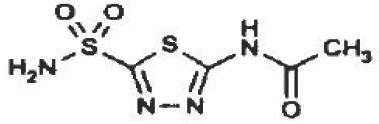
DESCRIPTIONAcetazolamide, an inhibitor of the enzyme carbonic anhydrase, is a white to faintly yellowish white crystalline, odorless powder, weakly acidic, very slightly soluble in water and slightly soluble ...
-
CLINICAL PHARMACOLOGYAcetazolamide is a potent carbonic anhydrase inhibitor, effective in the control of fluid secretion (e.g., some types of glaucoma), in the treatment of certain convulsive disorders (e.g. ...
-
INDICATIONS AND USAGEFor adjunctive treatment of: edema due to congestive heart failure; drug-induced edema; centrencephalic epilepsies (petit mal, unlocalized seizures); chronic simple (open-angle) glaucoma ...
-
CONTRAINDICATIONSAcetazolamide therapy is contraindicated in situations in which sodium and/or potassium blood serum levels are depressed, in cases of marked kidney and liver disease or dysfunction, in suprarenal ...
-
WARNINGSFatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis ...
-
PRECAUTIONSGeneral - Increasing the dose does not increase the diuresis and may increase the incidence of drowsiness and/or paresthesia. Increasing the dose often results in a decrease in diuresis. Under ...
-
ADVERSE REACTIONSAdverse reactions, occurring most often early in therapy, include paresthesias, particularly a "tingling" feeling in the extremities, hearing dysfunction or tinnitus, loss of appetite, taste ...
-
OVERDOSAGENo data are available regarding acetazolamide overdosage in humans as no cases of acute poisoning with this drug have been reported. Animal data suggest that acetazolamide is remarkably nontoxic ...
-
DOSAGE AND ADMINISTRATIONGlaucoma - Acetazolamide should be used as an adjunct to the usual therapy. The dosage employed in the treatment of - chronic simple (open-angle) glaucomaranges from 250 mg to 1 g of ...
-
HOW SUPPLIEDAcetazolamide Tablets USP are supplied as follows: 125 mg - White, round, scored in half, on one side, "T52" engraved on the other side. NDC 51672-4022-1 - Bottle of 100 - 250 mg ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 2624761 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: October, 2015 - 79429-1015-3
-
PRINCIPAL DISPLAY PANEL - 125 mg Tablet Bottle LabelNDC 51672-4022-1 - 100 Tablets - AcetaZOLAMIDE - Tablets USP, 125 mg - TARO - Keep this and all medications out of - the reach of children. Rx only
-
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Bottle LabelNDC 51672-4023-1 - 100 Tablets - AcetaZOLAMIDE - Tablets USP, 250 mg - TARO - Keep this and all medications out of - the reach of children. Rx only
-
INGREDIENTS AND APPEARANCEProduct Information