Label: NORTRIPTYLINE HYDROCHLORIDE capsule
- NDC Code(s): 51672-4001-1, 51672-4001-2, 51672-4001-5, 51672-4001-6, view more
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 4, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of nortriptyline hydrochloride or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Nortriptyline hydrochloride is not approved for use in pediatric patients (see WARNINGS, Clinical Worsening and Suicide Risk; PRECAUTIONS, Information for Patients, and PRECAUTIONS, Pediatric Use).
Close -
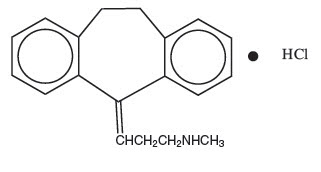
DESCRIPTIONNortriptyline Hydrochloride, USP is 1-propanamine, 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N-methyl, hydrochloride. The structural formula is as follows: C19H21N∙HCl MW ...
-
CLINICAL PHARMACOLOGYThe mechanism of mood elevation by tricyclic antidepressants is at present unknown. Nortriptyline hydrochloride is not a monoamine oxidase inhibitor. It inhibits the activity of such diverse ...
-
INDICATIONS AND USAGENortriptyline hydrochloride is indicated for the relief of symptoms of depression. Endogenous depressions are more likely to be alleviated than are other depressive states.
-
CONTRAINDICATIONSMonoamine Oxidase Inhibitors (MAOIs) The use of MAOIs intended to treat psychiatric disorders with nortriptyline hydrochloride or within 14 days of stopping treatment with nortriptyline ...
-
WARNINGSClinical Worsening and Suicide Risk - Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ...
-
PRECAUTIONSInformation for Patients - Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with ...
-
ADVERSE REACTIONSNote - Included in the following list are a few adverse reactions that have not been reported with this specific drug. However, the pharmacologic similarities among the tricyclic antidepressant ...
-
OVERDOSAGEDeaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and ...
-
DOSAGE AND ADMINISTRATIONNortriptyline hydrochloride is not recommended for children. Nortriptyline hydrochloride is administered orally in the form of capsules. Lower than usual dosages are recommended for elderly ...
-
HOW SUPPLIEDNortriptyline Hydrochloride Capsules USP, equivalent to 10 mg, 25 mg, 50 mg, and 75 mg base, are as follows: 10 mg: Opaque light green cap and body, imprinted "TARO" on the cap and "NTP10" on the ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 2624761 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Dispense with Medication Guide available at ...
-
Medication GuideNortriptyline Hydrochloride (nor trip' ti leen hye'' droe klor' ide) Capsules, USP - Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle LabelNDC 51672-4001-1 - 100 Capsules - DISPENSE WITH THE ACCOMPANYING - MEDICATION GUIDE - Nortriptyline HCl - Capsules, USP equivalent to - 10 mg base - Keep this and all medications - out of the reach of ...
-
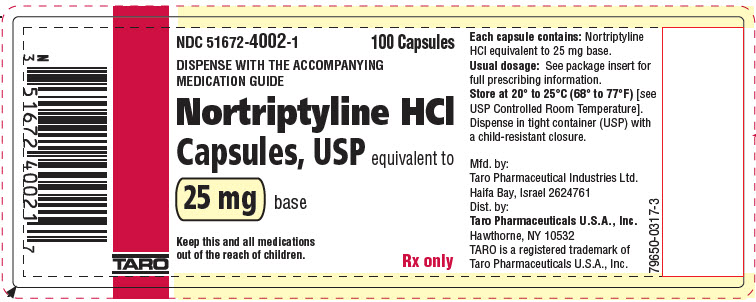
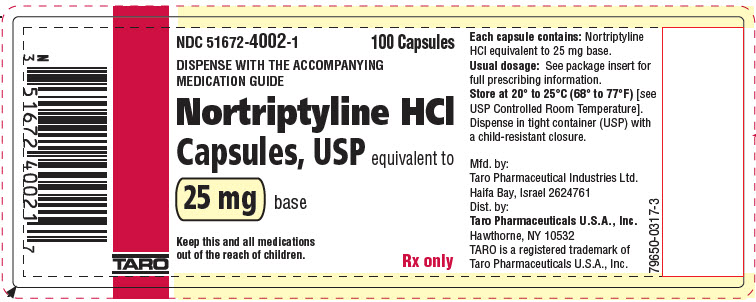
PRINCIPAL DISPLAY PANEL - 25 mg Capsule Bottle LabelNDC 51672-4002-1 - 100 Capsules - DISPENSE WITH THE ACCOMPANYING - MEDICATION GUIDE - Nortriptyline HCl - Capsules, USP equivalent to - 25 mg base - Keep this and all medications - out of the reach of ...
-
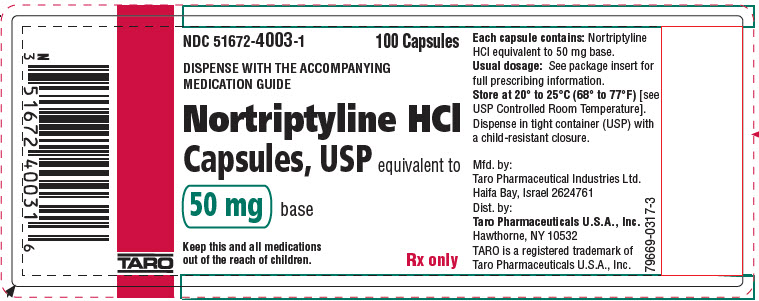
PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle LabelNDC 51672-4003-1 - 100 Capsules - DISPENSE WITH THE ACCOMPANYING - MEDICATION GUIDE - Nortriptyline HCl - Capsules, USP equivalent to - 50 mg base - Keep this and all medications - out of the reach of ...
-
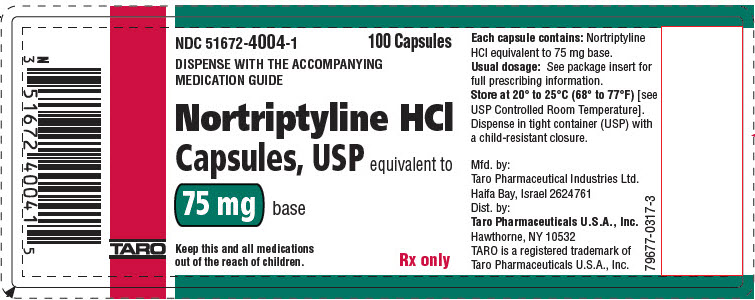
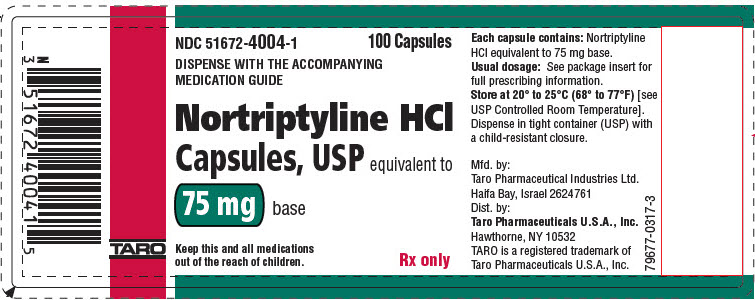
PRINCIPAL DISPLAY PANEL - 75 mg Capsule Bottle LabelNDC 51672-4004-1 - 100 Capsules - DISPENSE WITH THE ACCOMPANYING - MEDICATION GUIDE - Nortriptyline HCl - Capsules, USP equivalent to - 75 mg base - Keep this and all medications - out of the reach of ...
-
INGREDIENTS AND APPEARANCEProduct Information