Label: CLINDAMYCIN PHOSPHATE AND BENZOYL PEROXIDE gel
- NDC Code(s): 51672-1403-4
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLINDAMYCIN PHOSPHATE and BENZOYL PEROXIDE GEL safely and effectively. See full prescribing information for CLINDAMYCIN PHOSPHATE and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEClindamycin phosphate and benzoyl peroxide gel, 1.2%/3.75% is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
-
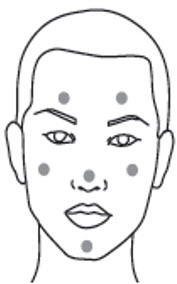
2 DOSAGE AND ADMINISTRATIONBefore applying clindamycin phosphate and benzoyl peroxide gel, wash the face gently with a mild soap, rinse with warm water, and pat the skin dry. Apply a pea-sized amount of clindamycin ...
-
3 DOSAGE FORMS AND STRENGTHSGel, 1.2%/3.75% Each gram of clindamycin phosphate and benzoyl peroxide gel contains 12 mg (1.2%) clindamycin phosphate, equivalent to 10 mg (1%) clindamycin, and 37.5 mg (3.75%) benzoyl peroxide ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Clindamycin phosphate and benzoyl peroxide gel is contraindicated in those individuals who have shown hypersensitivity to clindamycin, benzoyl peroxide, any components of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Colitis - Systemic absorption of clindamycin has been demonstrated following topical use of clindamycin. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been ...
-
6 ADVERSE REACTIONSThe following adverse reaction is described in more detail in the Warnings and Precautions section of the label: Colitis [see Warnings and Precautions (5.1)]. 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Erythromycin - Avoid using clindamycin phosphate and benzoyl peroxide gel in combination with topical or oral erythromycin-containing products due to its clindamycin component. In vitro ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on clindamycin phosphate and benzoyl peroxide gel use in pregnant women to evaluate a drug-associated risk of major birth defects ...
-
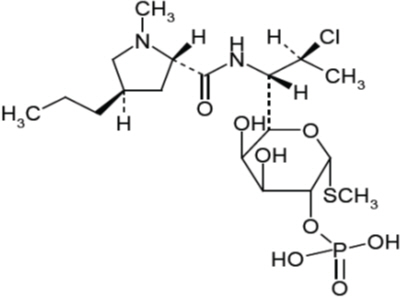
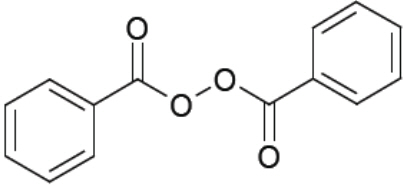
11 DESCRIPTIONClindamycin Phosphate and Benzoyl Peroxide Gel is a combination product with two active ingredients in a white to off-white, opaque, smooth, aqueous gel formulation intended for topical use ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clindamycin: Clindamycin is a lincosamide antibacterial [see Microbiology (12.4)]. Benzoyl Peroxide: Benzoyl peroxide is an oxidizing agent with bactericidal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity, and impairment of fertility testing of clindamycin phosphate and benzoyl peroxide gel have not been ...
-
14 CLINICAL STUDIESThe safety and efficacy of once-daily use of clindamycin phosphate and benzoyl peroxide gel was assessed in a 12-week multi-center, randomized, blinded trial in subjects 12 years and older with ...
-
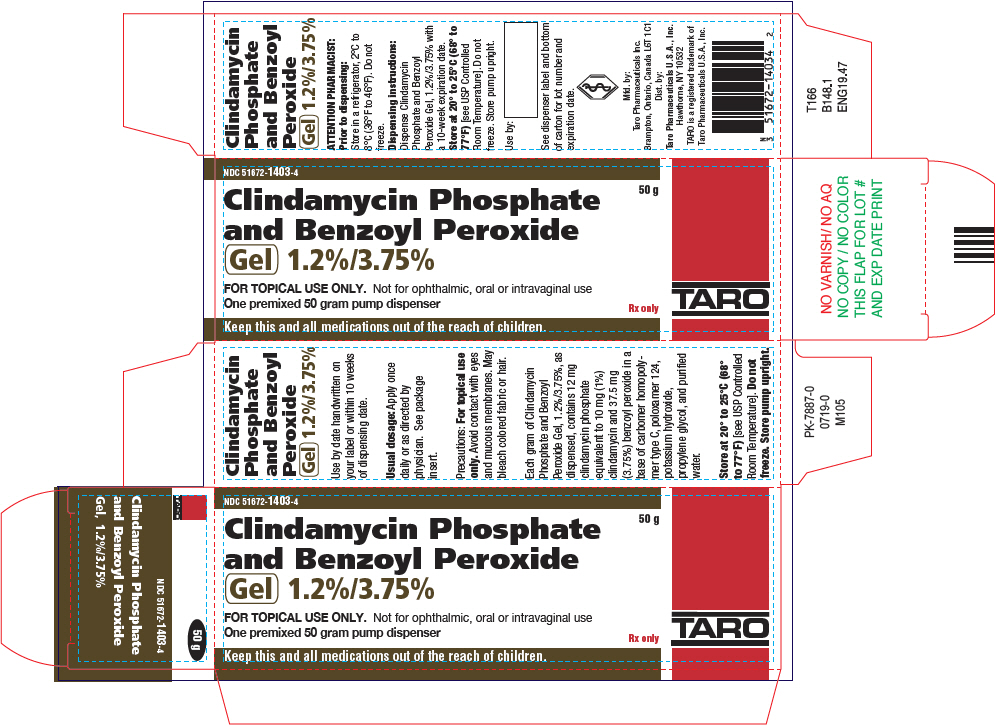
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Clindamycin Phosphate and Benzoyl Peroxide Gel 1.2%/3.75% is a white to off-white smooth gel supplied as a 50 g pump (NDC 51672-1403-4) 16.2 Dispensing Instructions ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Patients who develop allergic reactions, such as severe swelling or shortness of breath, should discontinue use ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Distributed by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: January 2023 - 5211506 - 25
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Clindamycin Phosphate - (klin'' da mye' sin fos' fate) and - Benzoyl Peroxide (BEN-zoe-il per-OX-ide) Gel, 1.2%/3.75% This Patient Information has been approved by the ...
-
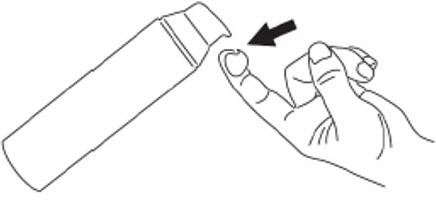
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - Clindamycin Phosphate - (klin'' da mye' sin fos' fate) and - Benzoyl Peroxide (BEN-zoe-il per-OX-ide) Gel, 1.2%/3.75% The Patient Information and Instructions for Use ...
-
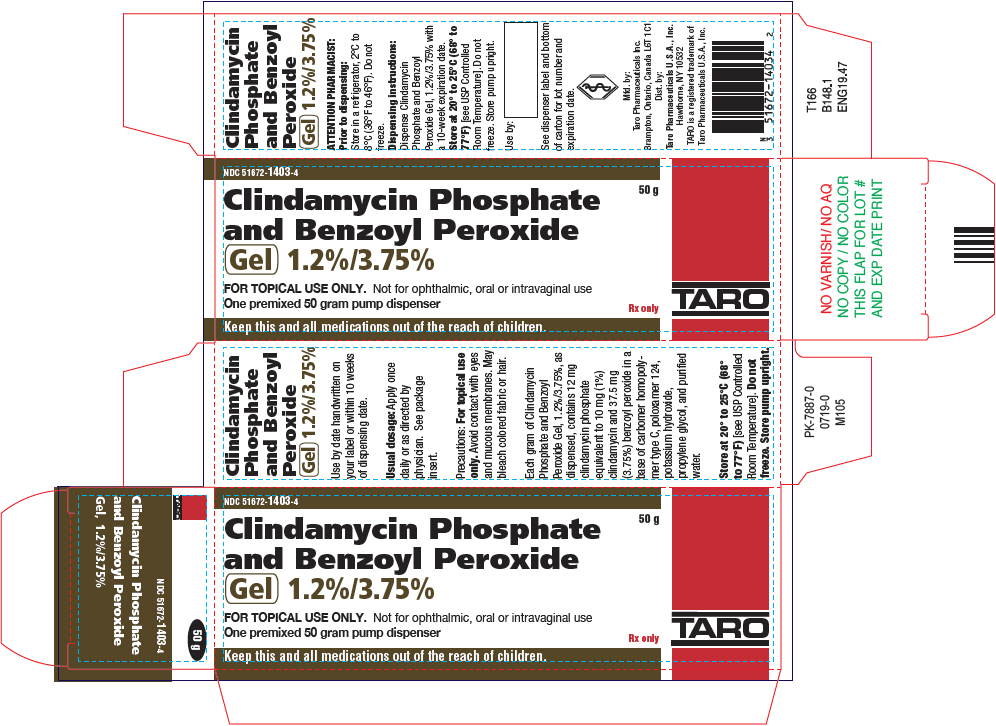
PRINCIPAL DISPLAY PANEL - 50 g Bottle CartonNDC 51672-1403-4 - Clindamycin Phosphate - and Benzoyl Peroxide - Gel 1.2%/3.75% 50 g - FOR TOPICAL USE ONLY. Not for ophthalmic, oral or intravaginal use - One premixed 50 gram pump dispenser - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information