Label: CLINDAMYCIN PHOSPHATE lotion
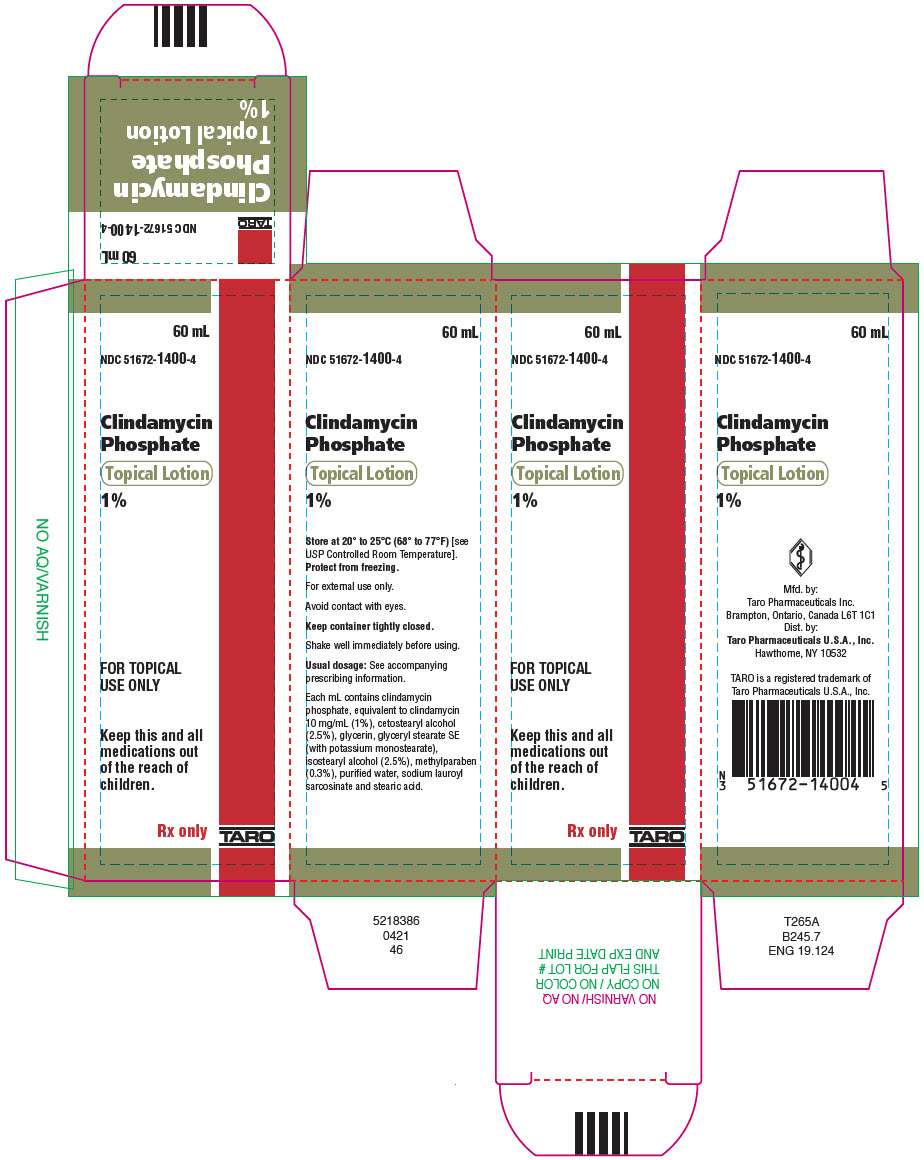
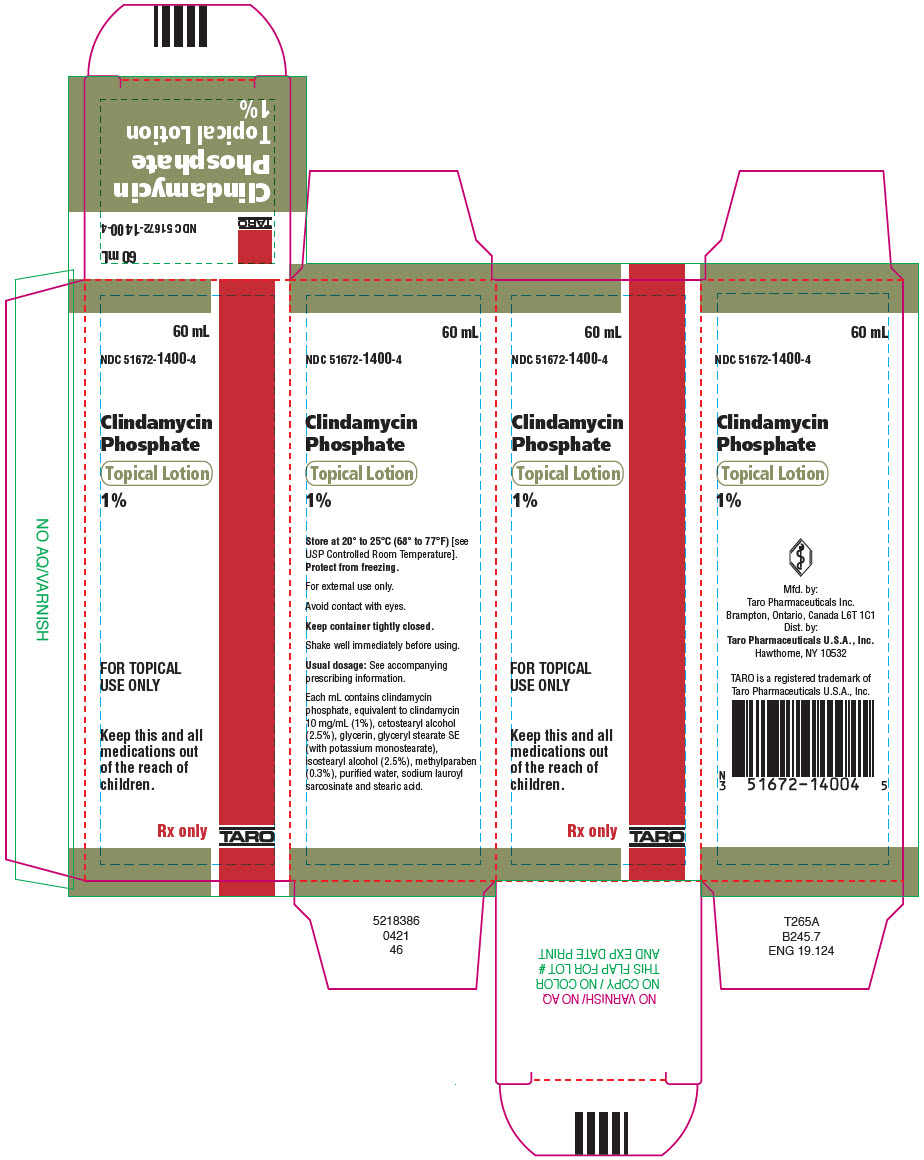
- NDC Code(s): 51672-1400-4
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor External Use
-
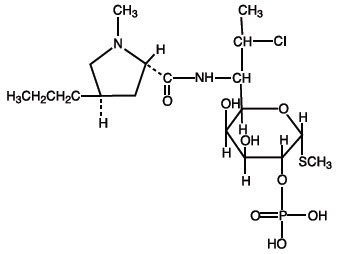
DESCRIPTIONClindamycin Phosphate Topical Lotion, 1% contains clindamycin phosphate, USP, at a concentration equivalent to 10 mg clindamycin per milliliter. Clindamycin phosphate is a water soluble ester of ...
-
CLINICAL PHARMACOLOGYMechanism of Action - The mechanism of action of clindamycin in treating acne vulgaris is unknown. Pharmacokinetics - Following multiple topical applications of clindamycin phosphate at a ...
-
INDICATIONS AND USAGEClindamycin phosphate topical lotion is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician should ...
-
CONTRAINDICATIONSClindamycin phosphate topical lotion is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional enteritis or ...
-
WARNINGSOrally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption of ...
-
PRECAUTIONSGeneral - Clindamycin phosphate topical lotion should be prescribed with caution in atopic individuals. Drug Interactions - Clindamycin has been shown to have neuromuscular blocking properties ...
-
ADVERSE REACTIONSIn 18 clinical studies of various formulations of clindamycin phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent ...
-
OVERDOSAGETopically applied clindamycin phosphate can be absorbed in sufficient amounts to produce systemic effects (see WARNINGS).
-
DOSAGE AND ADMINISTRATIONApply a thin film of clindamycin phosphate topical lotion twice daily to affected area. Shake well immediately before using. Keep all liquid dosage forms in containers tightly closed.
-
HOW SUPPLIEDClindamycin Phosphate Topical Lotion, 1% containing clindamycin phosphate equivalent to 10 mg clindamycin per milliliter is available in the following size: 60 mL plastic squeeze bottle – NDC ...
-
SPL UNCLASSIFIED SECTIONRx only - This product's label may have been updated. For current full prescribing information, please visit www.taro.com. Mfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T ...
-
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Carton60 mL - NDC 51672-1400-4 - Clindamycin - Phosphate - Topical Lotion - 1% FOR TOPICAL - USE ONLY - Keep this and all - medications out - of the reach of - children. Rx only - TARO
-
INGREDIENTS AND APPEARANCEProduct Information