Label: AZELAIC ACID gel
- NDC Code(s): 51672-1389-3
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AZELAIC ACID GEL safely and effectively. See full prescribing information for AZELAIC ACID GEL. AZELAIC ACID gel, for topical use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAzelaic acid gel, 15% is indicated for topical treatment of the inflammatory papules and pustules of mild to moderate rosacea. Limitations of Use - Although some reduction of erythema which was ...
-
2 DOSAGE AND ADMINISTRATIONCleanse affected area(s) using only very mild soaps or soapless cleansing lotion and pat dry with a soft towel before application of azelaic acid gel. Apply and gently massage a thin layer of ...
-
3 DOSAGE FORMS AND STRENGTHSAzelaic acid gel, 15% is a white to yellowish white opaque gel. Each gram of azelaic acid gel contains 0.15 gm of azelaic acid (15% w/w).
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Hypersensitivity reactions, including cases of angioedema, eye swelling, facial swelling, dyspnea, urticaria, and adverse skin reactions, have been reported during post ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Azelaic acid is minimally absorbed systemically following topical route of administration, and maternal use is not expected to result in fetal exposure to the ...
-
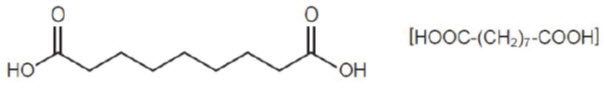
11 DESCRIPTIONAzelaic acid gel, 15%, is an aqueous gel which contains azelaic acid, a naturally-occurring saturated dicarboxylic acid. It is for topical use. Chemically, azelaic acid is 1,7-heptanedicarboxylic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism(s) by which azelaic acid interferes with the pathogenic events in rosacea are unknown. 12.2 Pharmacodynamics - The pharmacodynamics of azelaic acid in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year dermal mouse carcinogenicity study, azelaic acid pre-foam emulsion was administered twice daily to CD-1 mice at topical ...
-
14 CLINICAL STUDIESAzelaic acid gel was evaluated for the treatment of mild to moderate papulopustular rosacea in two multicenter, randomized, double-blind, vehicle-controlled, 12-week clinical trials having ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Azelaic acid gel, 15% is a white to yellowish white opaque gel supplied in a 50 g tube (NDC 51672-1389-3). Storage and Handling - Store at 20° to 25°C (68° to 77°F) [see USP ...
-
17 PATIENT COUNSELING INFORMATIONInform patients using azelaic acid gel of the following: Administration Instructions - For topical use only. Before applying azelaic acid gel, cleanse affected area(s) with a very mild soap or ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Distributed by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: December 2021 - 5216657 - 30
-
PRINCIPAL DISPLAY PANEL - 50 g Tube CartonNDC 51672-1389-3 - 50 g - Azelaic Acid - Gel - 15% For Topical Use Only - Not for oral, ophthalmic or intravaginal use - Rx only - Keep this and all medications out of the reach of ...
-
INGREDIENTS AND APPEARANCEProduct Information