Label: NAFTIFINE HYDROCHLORIDE cream
- NDC Code(s): 51672-1368-2, 51672-1368-3, 51672-1368-6
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 25, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NAFTIFINE HYDROCHLORIDE CREAM safely and effectively. See full prescribing information for NAFTIFINE HYDROCHLORIDE CREAM - NAFTIFINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENaftifine Hydrochloride Cream is indicated for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organism Trichophyton rubrum.
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. Naftifine Hydrochloride Cream is not for ophthalmic, oral, or intravaginal use. Apply a thin layer of Naftifine Hydrochloride Cream once-daily to the affected areas plus a ...
-
3 DOSAGE FORMS AND STRENGTHSEach gram of Naftifine Hydrochloride Cream contains 20 mg of naftifine hydrochloride (2%) in a white to off-white base.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Local Adverse Reactions - Discontinue treatment if irritation or sensitivity develops with the use of Naftifine Hydrochloride Cream. Direct patients to contact their physician if these ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with Naftifine Hydrochloride Cream in pregnant women to inform the drug-associated risk for major birth defects and miscarriage. In ...
-
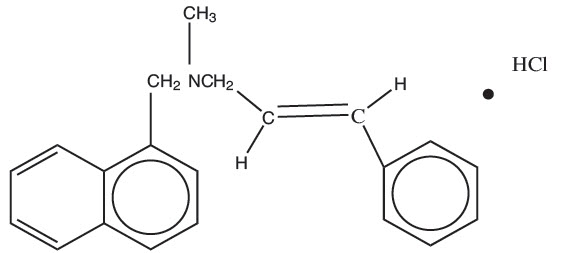
11 DESCRIPTIONNaftifine Hydrochloride Cream is a white to off-white cream for topical use only. Each gram of Naftifine Hydrochloride Cream contains 20 mg of naftifine hydrochloride (2%), a synthetic allylamine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Naftifine Hydrochloride Cream is a topical antifungal drug [see Clinical Pharmacology (12.4)] 12.2 Pharmacodynamics - The pharmacodynamics of Naftifine Hydrochloride ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year dermal carcinogenicity study, naftifine hydrochloride cream was administered to Sprague-Dawley rats at topical doses of 1% ...
-
14 CLINICAL STUDIES14.1 Tinea Cruris - Naftifine Hydrochloride Cream has been investigated for safety and efficacy in a randomized, double-blind, vehicle-controlled, multi-center trial in 146 subjects with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Naftifine Hydrochloride Cream USP, 2% is a white to off-white cream supplied in tubes in the following sizes: 30 g – NDC 51672-1368-2 - 45 g – NDC 51672-1368-6 - 60 g – NDC ...
-
17 PATIENT COUNSELING INFORMATIONInform patients that Naftifine Hydrochloride Cream is for topical use only. Naftifine Hydrochloride Cream is not intended for oral, intravaginal or ophthalmic use. If irritation or sensitivity ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Taro Pharmaceuticals Inc. Brampton, Ontario, Canada L6T 1C1 - Distributed by: Taro Pharmaceuticals U.S.A., Inc. Hawthorne, NY 10532 - Revised: May, 2018 - PK-7472-2 - 22
-
PRINCIPAL DISPLAY PANEL - 30 g Tube CartonNDC 51672-1368-2 - 30 g - Naftifine Hydrochloride - Cream USP, 2% FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE, ORAL OR INTRAVAGINAL USE - Rx only - Keep this and all medications out of the reach ...
-
INGREDIENTS AND APPEARANCEProduct Information