Label: ADAPALENE AND BENZOYL PEROXIDE gel
- NDC Code(s): 51672-1364-3, 51672-1364-6
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADAPALENE AND BENZOYL PEROXIDE GEL safely and effectively. See full prescribing information for ADAPALENE AND BENZOYL PEROXIDE GEL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAdapalene and benzoyl peroxide gel 0.1%/2.5% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONFor topical use only; adapalene and benzoyl peroxide gel 0.1%/2.5% is not for oral, ophthalmic, or intravaginal use. Apply a thin film of adapalene and benzoyl peroxide gel to affected areas of ...
-
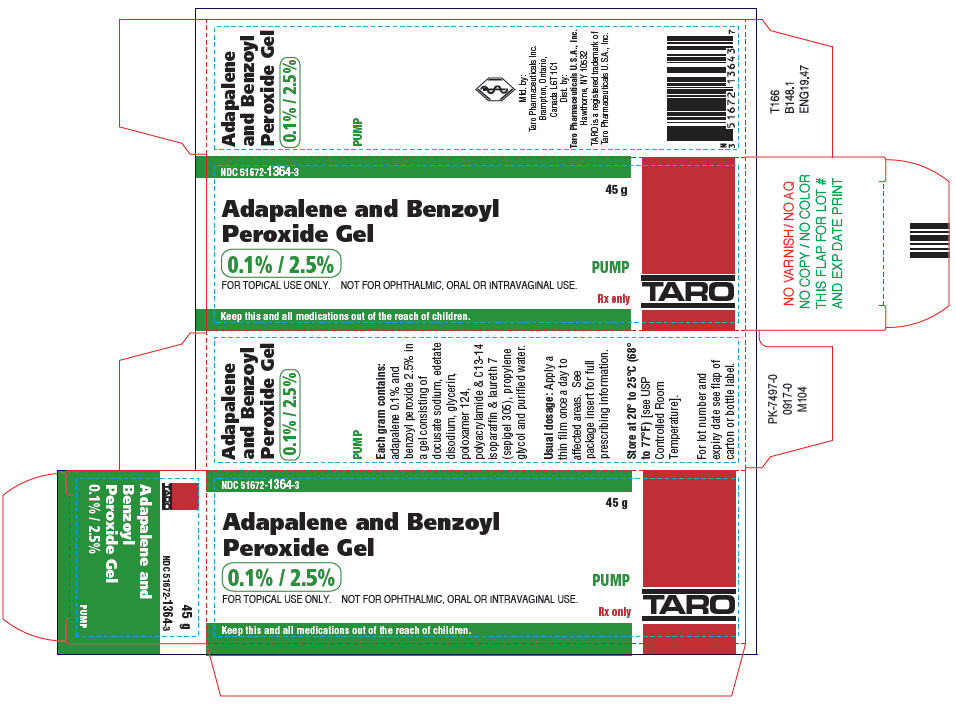
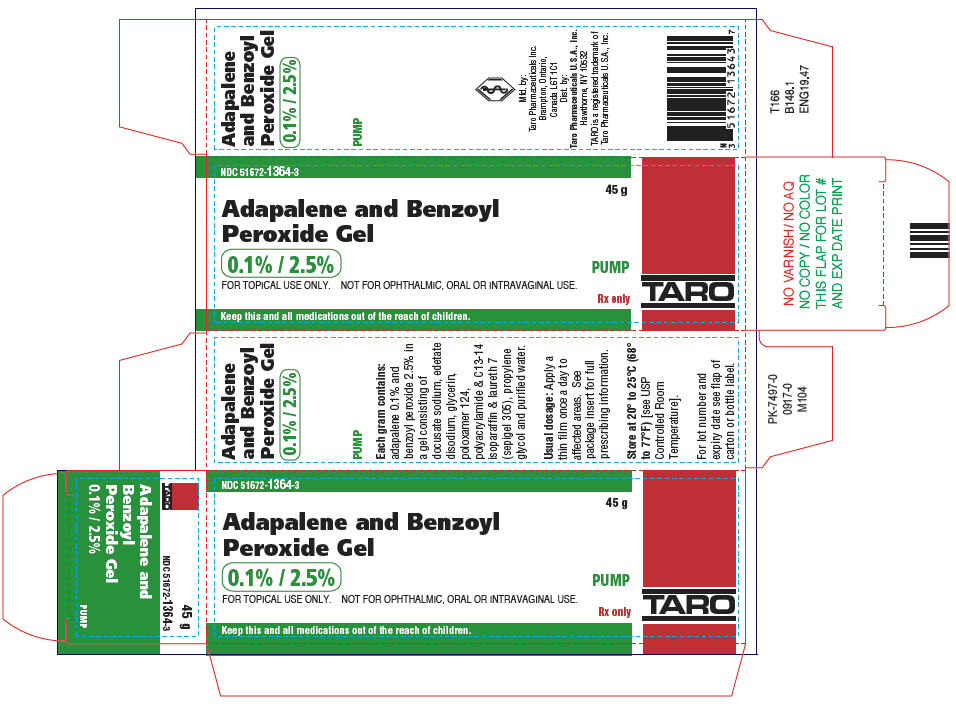
3 DOSAGE FORMS AND STRENGTHSEach gram of adapalene and benzoyl peroxide gel contains 1 mg (0.1%) adapalene and 25 mg (2.5%) benzoyl peroxide in a white to pale yellow, opaque gel.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Ultraviolet Light and Environmental Exposure - Exposure to sunlight, including sunlamps, should be minimized during the use of adapalene and benzoyl peroxide gel 0.1%/2.5%. Patients with high ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONSConcomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents. No ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C. There are no well-controlled trials in pregnant women treated with adapalene and benzoyl peroxide gel 0.1%/2.5%. Animal reproduction studies have not been ...
-
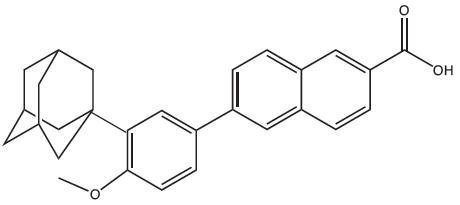
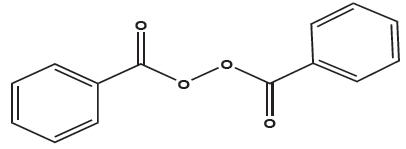
11 DESCRIPTIONAdapalene and Benzoyl Peroxide Gel, 0.1%/2.5% is a white to pale yellow, opaque gel for topical use containing adapalene 0.1% and benzoyl peroxide 2.5%. Adapalene, a synthetic retinoid, is a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Adapalene - Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity, photocarcinogenicity, genotoxicity, or fertility studies were conducted with adapalene and benzoyl peroxide ...
-
14 CLINICAL STUDIESThe safety and efficacy of adapalene and benzoyl peroxide gel applied once daily for the treatment of acne vulgaris were assessed in two 12-week, multicenter, controlled clinical studies of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAdapalene and Benzoyl Peroxide Gel 0.1%/2.5% is white to pale yellow opaque gel and is supplied as follows: 45 gram tubeNDC 51672-1364-6 - 45 gram pumpNDC 51672-1364-3 - Store at 20° to ...
-
17 PATIENT COUNSELING INFORMATION[See - FDA Approved Patient Labeling (Patient Information)] Information for Patients - Advise patients to cleanse the area to be treated with a mild or soapless cleanser; pat dry ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: February, 2020 - LPK-7373-1 54 ...
-
Patient Information
Adapalene and Benzoyl Peroxide Gel 0.1%/2.5%
Important:For use on the skin only (topical). Do not use adapalene and benzoyl peroxide gel in or on your mouth, eyes, or vagina. Read this Patient Information leaflet about adapalene and ...
-
PRINCIPAL DISPLAY PANEL - 45 g Bottle CartonNDC 51672-1364-3 - 45 g - Adapalene and Benzoyl - Peroxide Gel - 0.1% / 2.5% PUMP - FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL OR INTRAVAGINAL USE. Rx only - Keep this and all ...
-
INGREDIENTS AND APPEARANCEProduct Information