Label: FLUOCINOLONE ACETONIDE oil
- NDC Code(s): 51672-1356-8
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 16, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use fluocinolone acetonide topical oil safely and effectively. See full prescribing information for fluocinolone acetonide topical oil ...
-
Table of ContentsTable of Contents
-
SPL UNCLASSIFIED SECTIONFluocinolone Acetonide - Topical Oil, 0.01% (BODY OIL)
-
1 INDICATIONS AND USAGE1.1 Adult Patients with Atopic Dermatitis - Fluocinolone acetonide topical oil is indicated for the topical treatment of atopic dermatitis in adult patients. 1.2 Pediatric Patients with ...
-
2 DOSAGE AND ADMINISTRATIONFluocinolone acetonide topical oil is not for oral, ophthalmic, or intravaginal use. The dosing of fluocinolone acetonide topical oil is different for adult and pediatric patients. 2.1 Adult ...
-
3 DOSAGE FORMS AND STRENGTHSFluocinolone acetonide topical oil, 0.01% (Body Oil) is supplied in bottles containing 4 fluid ounces.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Hypothalamic-Pituitary-Adrenal Axis Suppression - Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to ...
-
10 OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects, including under conditions of normal use. [See Warnings and Precautions (5.1) and Use in ...
-
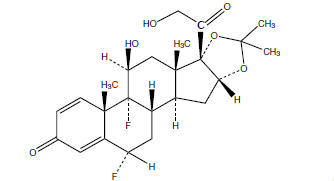
11 DESCRIPTIONFluocinolone acetonide topical oil, 0.01% (Body Oil) contains fluocinolone acetonide [(6α, 11β,16α)-6,9-difluoro-11,21-dihydroxy-16,17 [(1-methylethylidene)bis(oxy)]-pregna-1,4 diene-3,20- dione ...
-
12 CINICAL PHARMACOLOGY12.1 Mechanism of Action - Like other topical corticosteroids, fluocinolone acetonide has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, mutagenesis, impairment of fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of fluocinolone ...
-
16 HOW SUPPLIED / STORAGE AND HANDLINGFluocinolone acetonide topical oil is supplied in bottles containing 4 fluid ounces. It is labeled as Body Oil (NDC 51672-1356-8). Storage: Store at 20° to 25°C (68° to 77°F) [see USP ...
-
17 PATIENT COUNSELING INFORMATION17.1 Instructions - Fluocinolone acetonide topical oil should be used as directed by the physician. It is for external use only. Avoid contact with the eyes. In case of contact, wash eyes ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: June 2020 - PK-6468-1 50
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 118.28 mL Bottle Carton - 4 fl. oz. Net Contents 118.28 mL - NDC 51672-1356-8 - Fluocinolone - Acetonide - Topical Oil - 0.01% (BODY OIL) For Topical Use Only - Not for ...
-
INGREDIENTS AND APPEARANCEProduct Information