Label: CICLOPIROX OLAMINE cream
- NDC Code(s): 51672-1318-1, 51672-1318-2, 51672-1318-8
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR DERMATOLOGIC USE ONLY - NOT FOR OPHTHALMIC USE
-
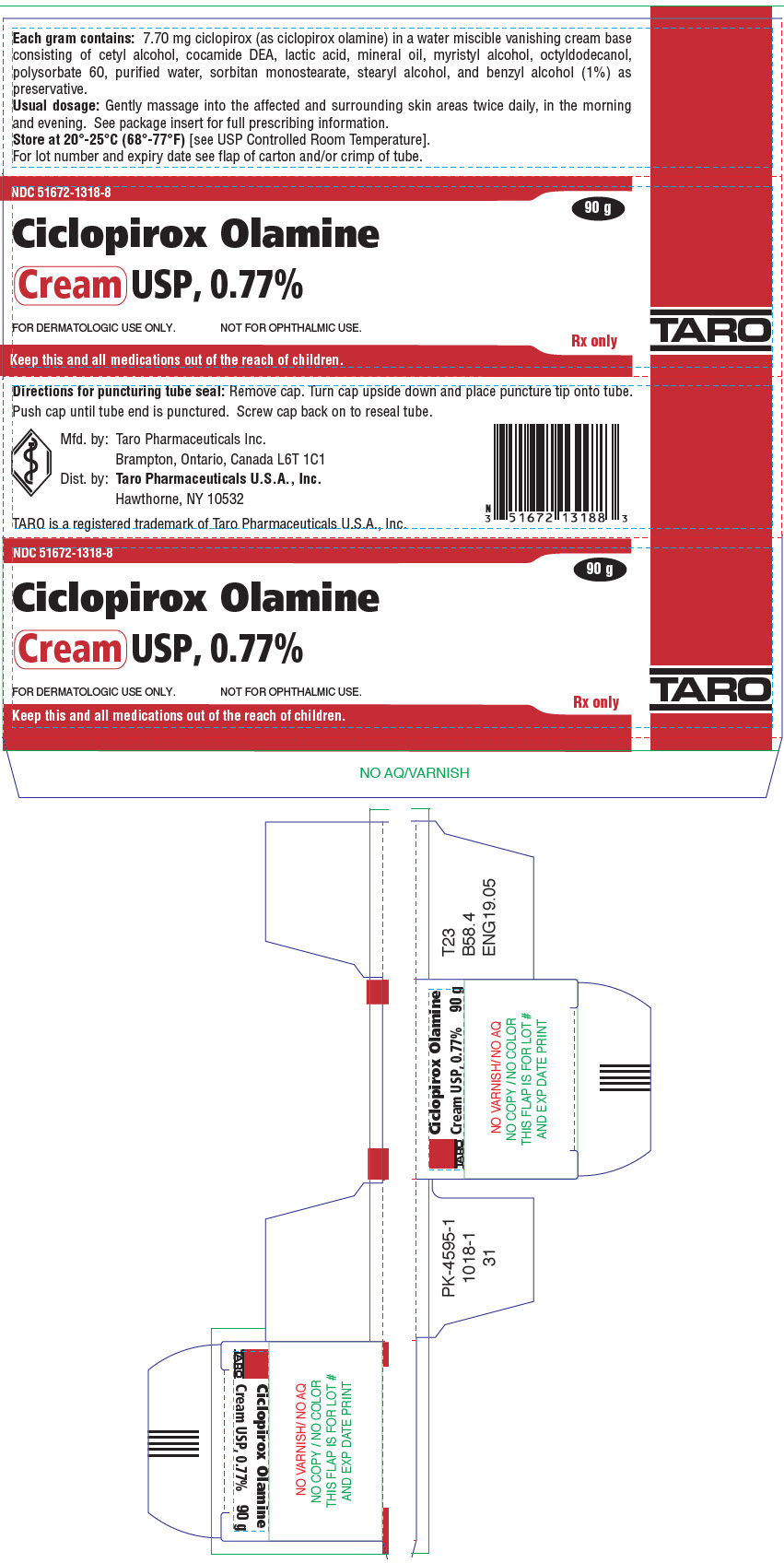
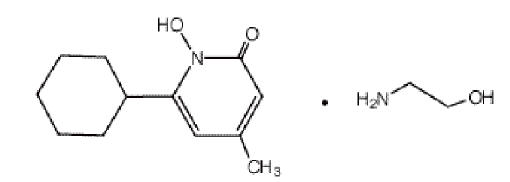
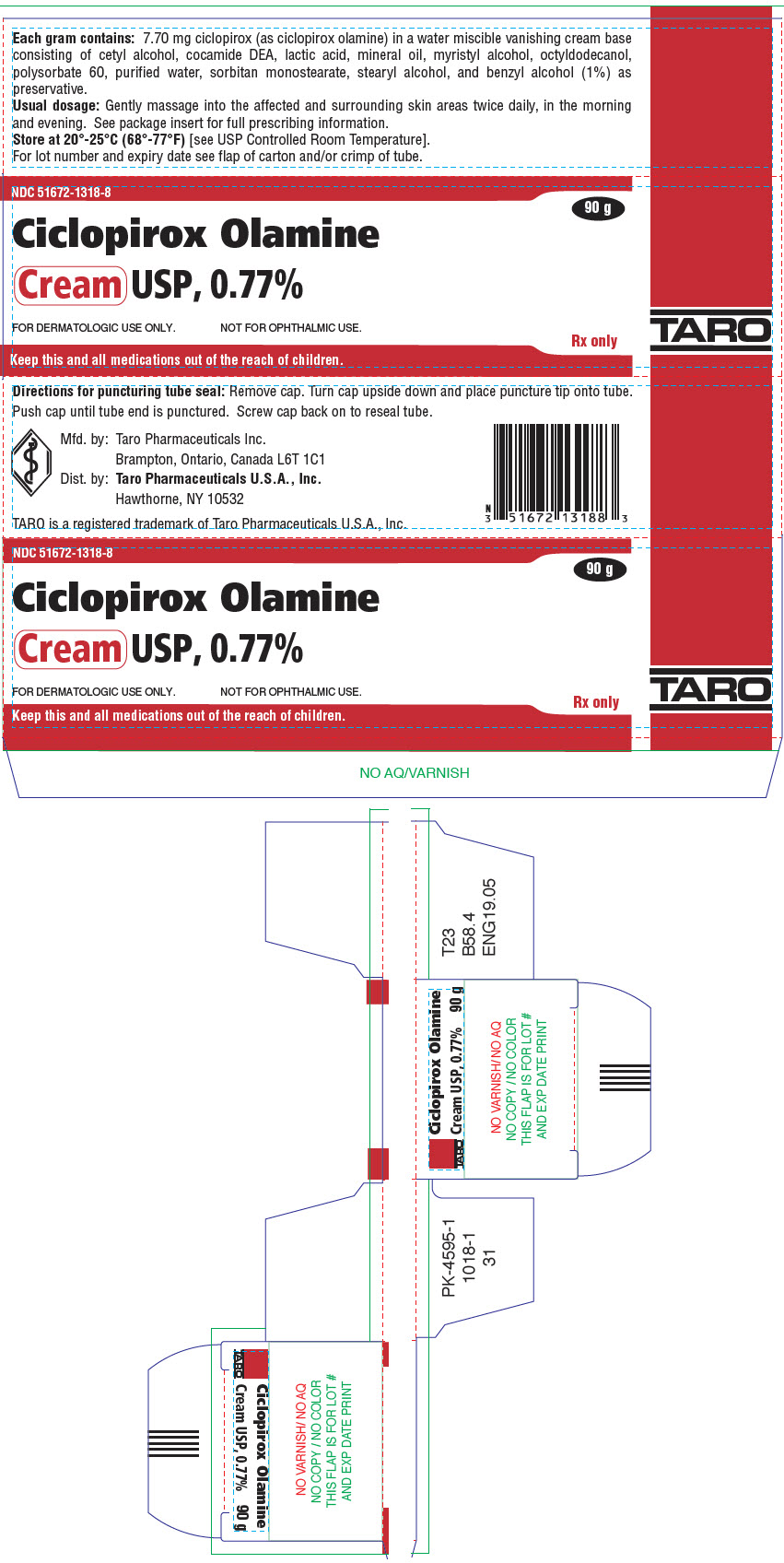
DESCRIPTIONCiclopirox Olamine Cream USP, 0.77% is for topical use. Each gram of ciclopirox olamine cream USP contains 7.70 mg of ciclopirox (as ciclopirox olamine) in a water miscible vanishing cream base ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Ciclopirox is a hydroxypyridone antifungal agent that acts by chelation of polyvalent cations (Fe - 3+or Al - 3+), resulting in the inhibition of the metal-dependent ...
-
INDICATIONS AND USAGECiclopirox olamine cream is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to - Trichophyton rubrum, Trichophyton ...
-
CONTRAINDICATIONSCiclopirox olamine cream is contraindicated in individuals who have shown hypersensitivity to any of its components.
-
WARNINGSCiclopirox olamine cream is not for ophthalmic use. Keep out of reach of children.
-
PRECAUTIONSIf a reaction suggesting sensitivity or chemical irritation should occur with the use of ciclopirox olamine cream, treatment should be discontinued and appropriate therapy ...
-
ADVERSE REACTIONSIn all controlled clinical studies with 514 patients using ciclopirox olamine cream and in 296 patients using the vehicle cream, the incidence of adverse reactions was low. This included pruritus ...
-
DOSAGE AND ADMINISTRATIONGently massage ciclopirox olamine cream into the affected and surrounding skin areas twice daily, in the morning and evening. Clinical improvement with relief of pruritus and other symptoms ...
-
HOW SUPPLIEDCiclopirox Olamine Cream USP, 0.77% is supplied in 15 g (NDC 51672-1318-1), 30 g (NDC 51672-1318-2) and 90 g (NDC 51672-1318-8) tubes. Store at 20°-25°C (68°-77°F)[see USP Controlled Room ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: July 2013 - PK-4597-1 226
-
PRINCIPAL DISPLAY PANEL - 90 g Tube CartonNDC 51672-1318-8 - Ciclopirox Olamine - Cream USP, 0.77% FOR DERMATOLOGIC USE ONLY. NOT FOR OPHTHALMIC USE. Keep this and all medications out of the reach of children. 90 g - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information