Label: MUPIROCIN ointment
- NDC Code(s): 51672-1312-0, 51672-1312-1, 51672-1312-2
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MUPIROCIN OINTMENT safely and effectively. See full prescribing information for MUPIROCIN OINTMENT. MUPIROCIN ointment, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMupirocin Ointment USP, 2% is indicated for the topical treatment of impetigo due to susceptible isolates of Staphylococcus aureus (S. aureus) and Streptococcus pyogenes (S. pyogenes).
-
2 DOSAGE AND ADMINISTRATIONFor Topical Use Only. Apply a small amount of mupirocin ointment, with a cotton swab or gauze pad, to the affected area 3 times daily for up to 10 days. Cover the treated area with gauze dressing ...
-
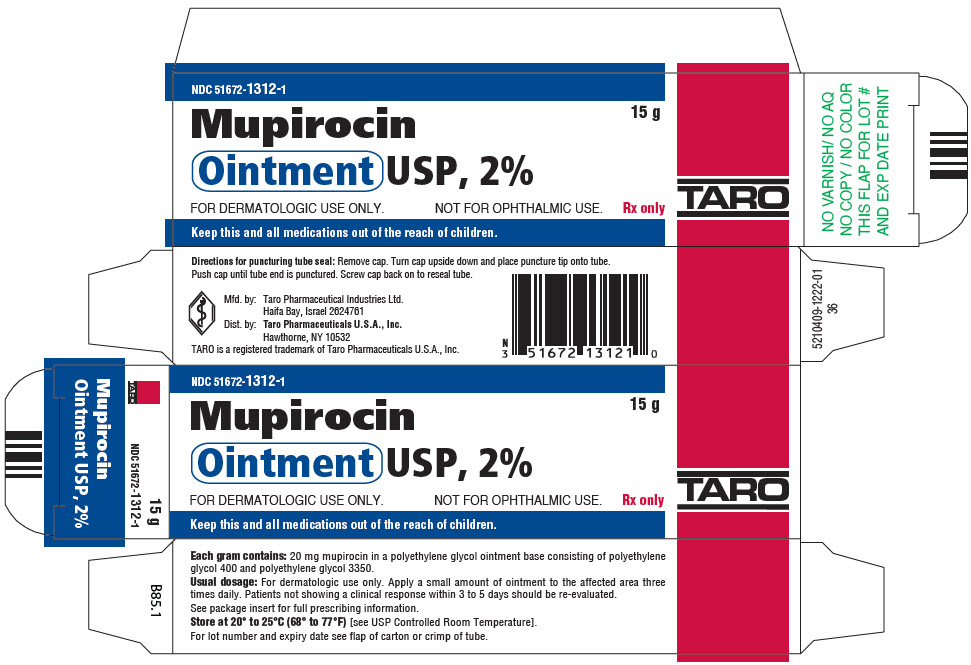
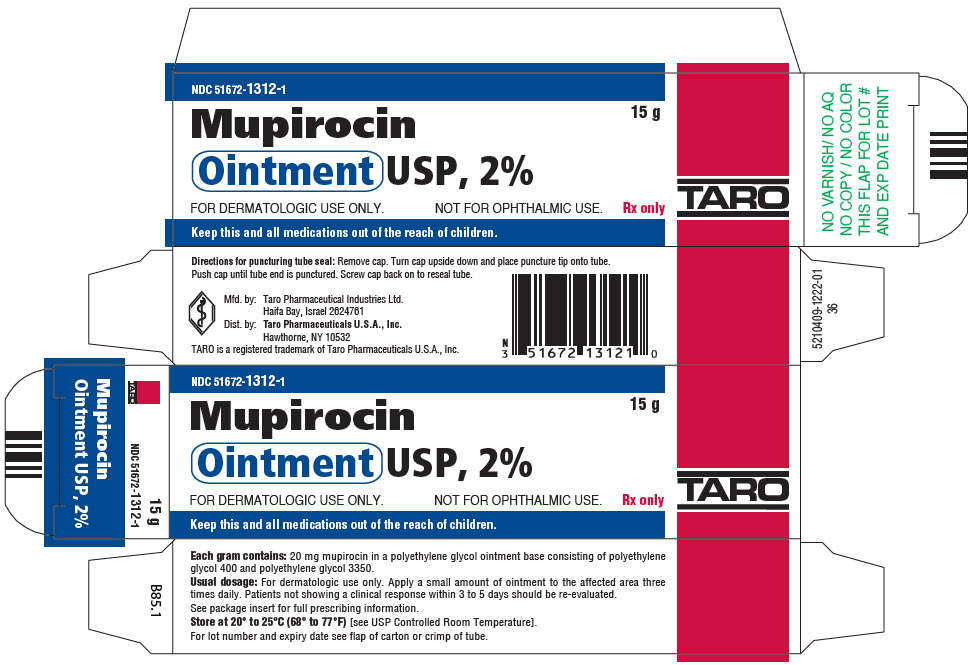
3 DOSAGE FORMS AND STRENGTHSEach gram of mupirocin ointment contains 20 mg mupirocin in a water-miscible ointment base supplied in 15-gram, 22-gram and 30-gram tubes.
-
4 CONTRAINDICATIONSMupirocin ointment is contraindicated in patients with known hypersensitivity to mupirocin or any of the excipients of mupirocin ointment.
-
5 WARNINGS AND PRECAUTIONS5.1 Severe Allergic Reactions - Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash, have been reported in patients treated with formulations of ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Severe Allergic Reactions [see Warnings and Precautions (5.1)] Eye Irritation [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient human data to establish whether there is a drug-associated risk with mupirocin ointment in pregnant women. Systemic absorption of ...
-
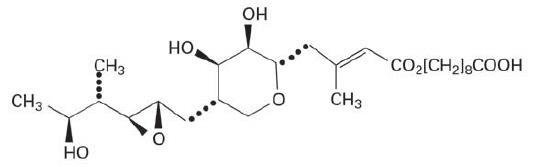
11 DESCRIPTIONMupirocin Ointment USP, 2% contains the RNA synthetase inhibitor antibacterial, mupirocin. The chemical name is (E)-(2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-epoxy-5-hydroxy-4-methylhexyl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mupirocin is an RNA synthetase inhibitor antibacterial [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption - Application of 14C-labeled mupirocin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential of mupirocin have not been conducted. Results of the following studies ...
-
14 CLINICAL STUDIESThe efficacy of topical mupirocin ointment in impetigo was tested in 2 trials. In the first, subjects with impetigo were randomized to receive either mupirocin ointment or vehicle placebo 3 times ...
-
15 REFERENCESClinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement. CLSI document M100-S26. Clinical and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach gram of mupirocin ointment contains 20 mg mupirocin in a water-miscible ointment base. Mupirocin Ointment USP, 2% is supplied as follows: 15-g tubeNDC 51672-1312-1 - 22-g tubeNDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Advise the patient to administer mupirocin ointment as follows: Use mupirocin ointment only as directed by ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: September, 2017 - PK-4402-5 - 5 - PHARMACIST-DETACH HERE ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION MUPIROCIN (mue pir`oh sin) OINTMENT - This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: September, 2017 - PK-4402-5 - 5 - What ...
-
PRINCIPAL DISPLAY PANEL - 15 g Tube CartonNDC 51672-1312-1 - 15 g - Mupirocin - Ointment USP, 2% FOR DERMATOLOGIC USE ONLY. NOT FOR OPHTHALMIC USE. Rx only - TARO - Keep this and all medications out of the reach of children.
-
INGREDIENTS AND APPEARANCEProduct Information