Label: BETAMETHASONE DIPROPIONATE cream, augmented
- NDC Code(s): 51672-1310-1, 51672-1310-2, 51672-1310-3
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 6, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BETAMETHASONE DIPROPIONATE CREAM (augmented) safely and effectively. See full prescribing information for BETAMETHASONE DIPROPIONATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBetamethasone dipropionate cream (augmented) is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 13 ...
-
2 DOSAGE AND ADMINISTRATIONApply a thin film of betamethasone dipropionate cream (augmented) to the affected skin areas once or twice daily. Therapy should be discontinued when control is achieved. Betamethasone ...
-
3 DOSAGE FORMS AND STRENGTHSCream, 0.05%. Each gram of betamethasone dipropionate cream USP (augmented), 0.05% contains 0.64 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone) in a white cream base.
-
4 CONTRAINDICATIONSBetamethasone dipropionate cream (augmented), is contraindicated in patients who are hypersensitive to betamethasone dipropionate, to other corticosteroids, or to any ingredient in this ...
-
5 WARNINGS AND PRECAUTIONS5.1 Effects on Endocrine System - Betamethasone dipropionate cream (augmented) can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on betamethasone dipropionate cream (augmented) use in pregnant women to identify a drug-associated risk of major birth defects ...
-
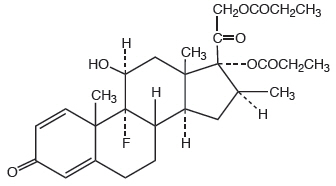
11 DESCRIPTIONBetamethasone dipropionate cream USP (augmented), 0.05% contains betamethasone dipropionate USP, a synthetic adrenocorticosteroid, for topical use in a cream base. Betamethasone, an analog of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of betamethasone ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of betamethasone dipropionate. Betamethasone ...
-
14 CLINICAL STUDIESThe safety and efficacy of betamethasone dipropionate cream (augmented) for the treatment of corticosteroid-responsive dermatoses have been established in two randomized and active controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGBetamethasone dipropionate cream USP (augmented), 0.05% is a white cream supplied in 15 g (NDC 51672-1310-1), 30 g (NDC 51672-1310-2) and 50 g (NDC 51672-1310-3) tubes. Store at 20° to 25°C ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Inform patients of the following: Discontinue therapy when control is achieved, unless directed otherwise by ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: December 2020 - 5212541 53
-
PATIENT PACKAGE INSERTPatient Information - Betamethasone Dipropionate - (bay'' ta meth' a sone dye proe' pee oh nate) Cream (Augmented) This Patient Information has been approved by the U.S. Food and Drug ...
-
PRINCIPAL DISPLAY PANEL - 15 g Tube CartonNDC 51672-1310-1 - 15 g - Betamethasone Dipropionate - Cream USP (Augmented), 0.05%* *Strength expressed as betamethasone - FOR DERMATOLOGIC USE ONLY. NOT FOR OPHTHALMIC USE. Rx only - Keep this and all ...
-
INGREDIENTS AND APPEARANCEProduct Information