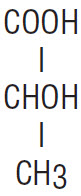Label: AMMONIUM LACTATE cream
- NDC Code(s): 51672-1301-0, 51672-1301-2, 51672-1301-4
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only. For Dermatological Use only. Not for Ophthalmic, Oral or Intravaginal Use.
-
DESCRIPTION* Ammonium Lactate Cream, 12% specially formulates 12% lactic acid, as ammonium lactate to provide a cream pH of 4.5 to 5.5. Ammonium Lactate Cream, 12% also contains cetyl alcohol, glycerin ...
-
CLINICAL PHARMACOLOGYLactic acid is an alpha-hydroxy acid. It is a normal constituent of tissues and blood. The alpha-hydroxy acids (and their salts) are felt to act as humectants when applied to the skin. This ...
-
INDICATIONS AND USAGEAmmonium Lactate Cream, 12% is indicated for the treatment of ichthyosis vulgaris and xerosis.
-
CONTRAINDICATIONSAmmonium Lactate Cream, 12% is contraindicated in those patients with a history of hypersensitivity to any of the label ingredients.
-
WARNINGSun exposure to areas of the skin treated with Ammonium Lactate Cream, 12% should be minimized or avoided (see - PRECAUTIONSsection). The use of Ammonium Lactate Cream, 12% should be ...
-
PRECAUTIONSGeneral - For external use only. Stinging or burning may occur when applied to skin with fissures, erosions, or that is otherwise abraded (for example, after shaving the legs). Caution is advised ...
-
ADVERSE REACTIONSIn controlled clinical trials of patients with ichthyosis vulgaris, the most frequent adverse reactions in patients treated with Ammonium Lactate Cream, 12% were rash (including erythema and ...
-
DOSAGE AND ADMINISTRATIONApply to the affected areas and rub in thoroughly. Use twice daily or as directed by a physician.
-
HOW SUPPLIEDAmmonium Lactate Cream, 12% is available as follows: 1-140 gram laminate tube NDC 51672-1301-2 - 2-140 gram laminate tubes NDC 51672-1301-4 - 385 gram plastic bottle NDC ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc. Brampton, Ontario - Canada L6T 1C1 - Dist. by: Taro Pharmaceuticals - U.S.A., Inc. Hawthorne, NY 10532 - Revised: October ...
-
PRINCIPAL DISPLAY PANEL - 385 g Bottle LabelNDC 51672-1301-4 - Ammonium - Lactate - Cream 12%*
-
INGREDIENTS AND APPEARANCEProduct Information