Label: CLOBETASOL PROPIONATE gel
CLOBETASOL PROPIONATE cream
CLOBETASOL PROPIONATE ointment
- NDC Code(s): 51672-1258-1, 51672-1258-2, 51672-1258-3, 51672-1258-6, view more
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR DERMATOLOGIC USE ONLY - NOT FOR OPHTHALMIC, ORAL OR INTRAVAGINAL USE
-
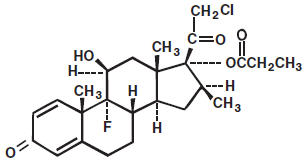
DESCRIPTIONClobetasol propionate gel, cream and ointment contain the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone ...
-
CLINICAL PHARMACOLOGYLike other topical corticosteroids, clobetasol propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical ...
-
INDICATIONS AND USAGEClobetasol propionate gel, cream and ointment are super-high potency corticosteroid formulations indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid ...
-
CONTRAINDICATIONSClobetasol propionate gel, cream and ointment are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparations.
-
PRECAUTIONSGeneral - Clobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at doses as low as 2 g per day. Systemic absorption of topical ...
-
ADVERSE REACTIONSIn a controlled clinical trial with clobetasol propionate gel, the only reported adverse reaction that was considered to be drug related was a report of burning sensation (1.8% of treated ...
-
OVERDOSAGETopically applied clobetasol propionate gel, cream or ointment can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
-
DOSAGE AND ADMINISTRATIONApply a thin layer of clobetasol propionate gel, cream or ointment to the affected skin areas twice daily and rub in gently and completely. (See INDICATIONS AND USAGE.) Clobetasol propionate gel ...
-
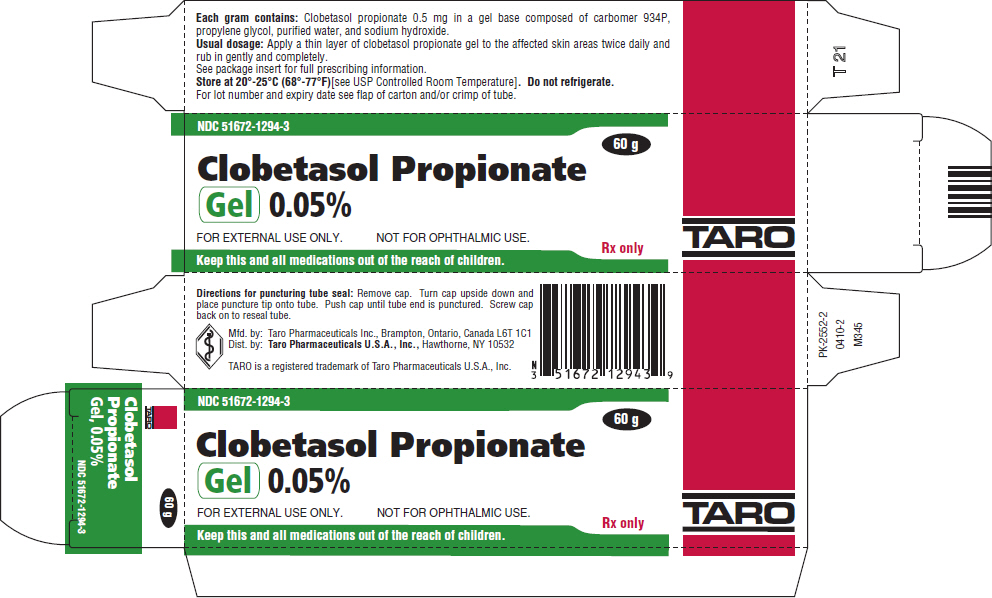
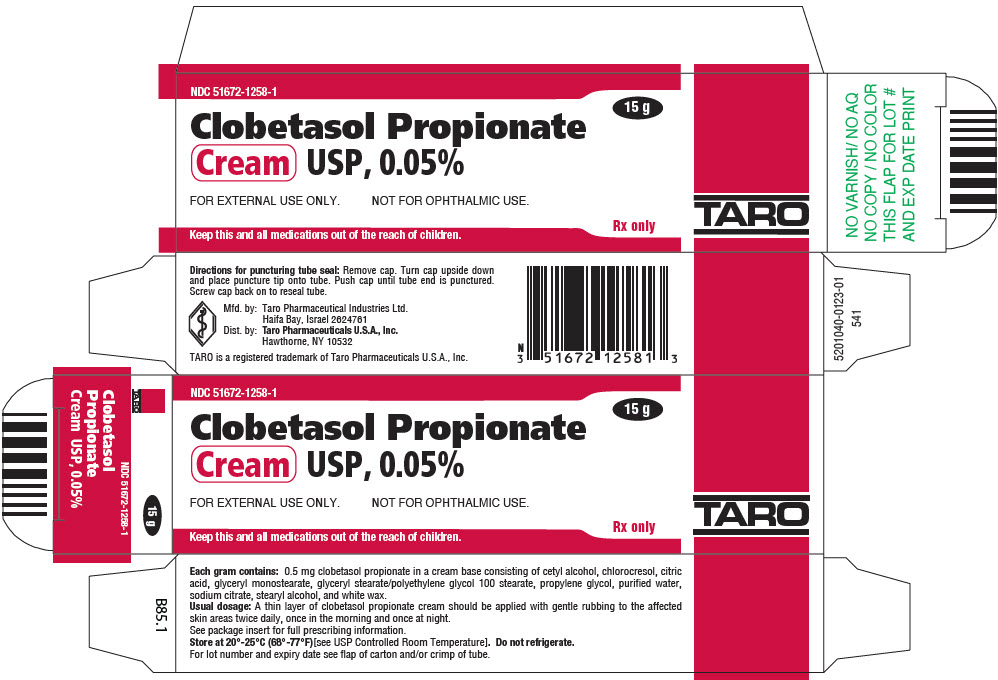
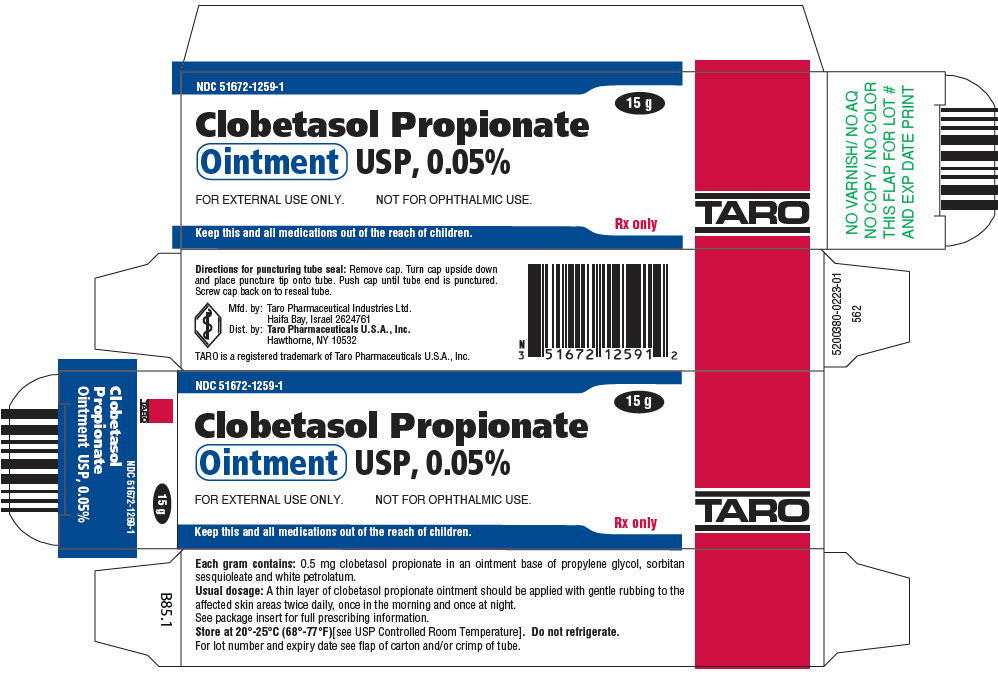
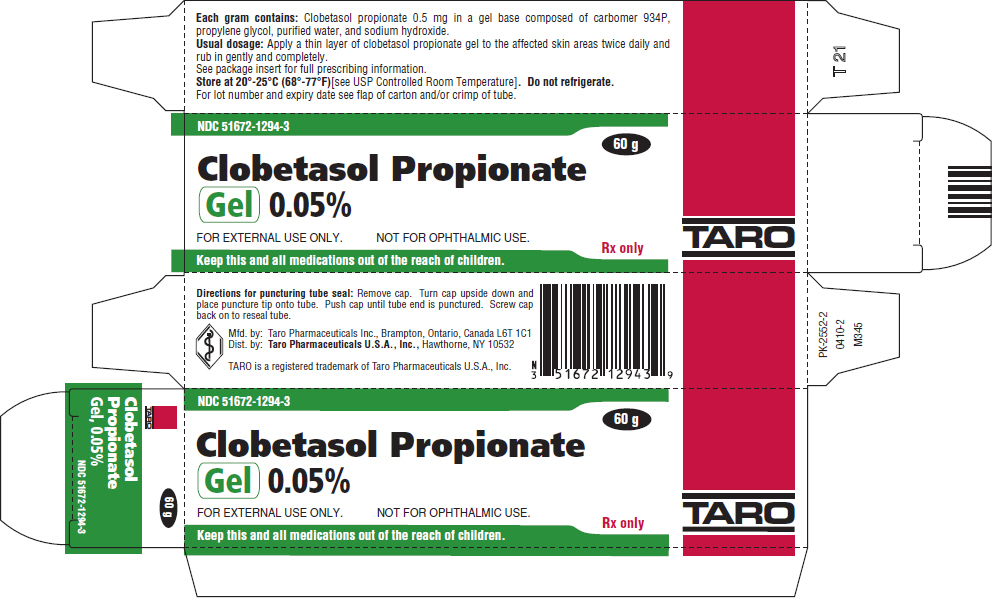
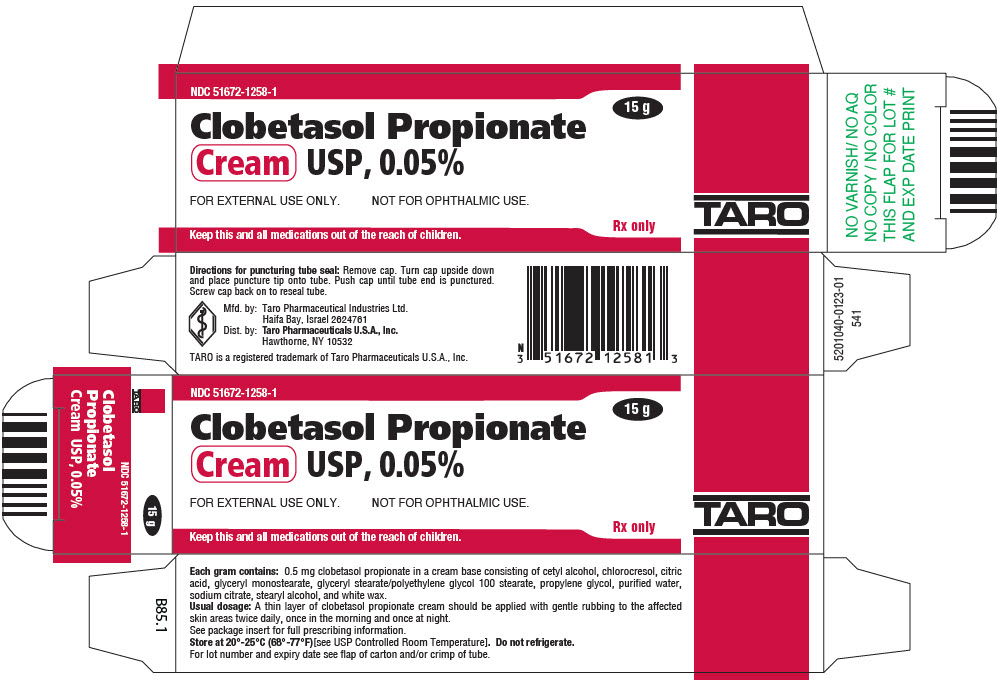
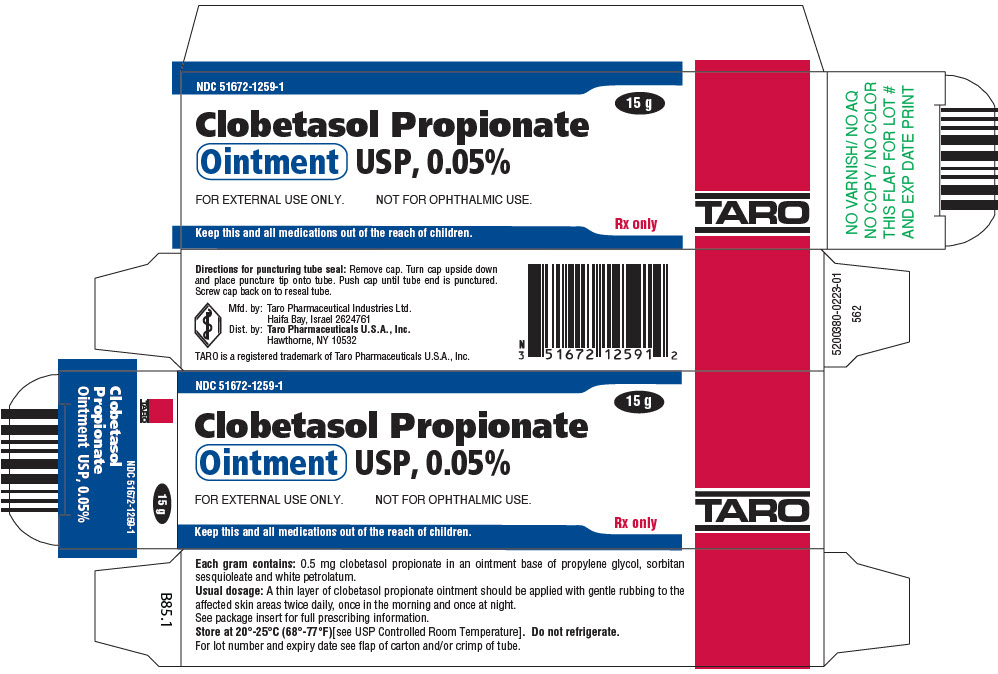
HOW SUPPLIEDClobetasol Propionate Gel, 0.05% is supplied in tamper-evident tubes:15 g (NDC 51672-1294-1), 30 g (NDC 51672-1294-2), and 60 g (NDC 51672-1294-3). Clobetasol Propionate Cream USP, 0.05% is ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Dist by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - Revised: October, 2019 - PK-6540-2 11
-
PRINCIPAL DISPLAY PANEL - 60 g Tube CartonNDC 51672-1294-3 - Clobetasol Propionate - Gel 0.05% FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. Keep this and all medications out of the reach of children. 60 g - Rx only - TARO
-
PRINCIPAL DISPLAY PANEL - 15 g Tube CartonNDC 51672-1258-1 - 15 g - Clobetasol Propionate - Cream USP, 0.05% FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. Keep this and all medications out of the reach of children. Rx only - TARO
-
PRINCIPAL DISPLAY PANEL - 15 g Ointment Tube CartonNDC 51672-1259-1 - 15 g - Clobetasol Propionate - Ointment USP, 0.05% FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. Keep this and all medications out of the reach of children. Rx only - TARO
-
INGREDIENTS AND APPEARANCEProduct Information