Label: SODIUM POLYSTYRENE SULFONATE powder, for suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 51293-831-97 - Packager: ECI Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SODIUM POLYSTYRENE SULFONATE POWDER FOR SUSPENSION safely and effectively. See full prescribing information for SODIUM POLYSTYRENE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESodium polystyrene sulfonate is indicated for the treatment of hyperkalemia. Limitation of Use: Sodium polystyrene sulfonate should not be used as an emergency treatment for life-threatening ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Information - Administer Sodium polystyrene sulfonate at least 3 hours before or 3 hours after other oral medications. Patients with gastroparesis may require a 6 hour separation [see ...
-
3 DOSAGE FORMS AND STRENGTHSSodium polystyrene sulfonate is a cream to light brown, finely ground powder and is available in 453.6 g jars.
-
4 CONTRAINDICATIONSSodium polystyrene sulfonate is contraindicated in patients with the following conditions: Hypersensitivity to polystyrene sulfonate resins - Obstructive bowel disease - Neonates with reduced gut ...
-
5 WARNINGS AND PRECAUTIONS5.1 Intestinal Necrosis - Cases of intestinal necrosis, some fatal, and other serious gastrointestinal adverse events (bleeding, ischemic colitis, perforation) have been reported in association ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed elsewhere in the labeling: Intestinal Necrosis [see Warnings and Precautions (5.1)] Electrolyte Disturbances [see Warnings and Precautions (5.2 ...
-
7 DRUG INTERACTIONS7.1 General Interactions - No formal drug interaction studies have been conducted in humans. Sodium polystyrene sulfonate has the potential to bind other drugs. In in vitro binding studies ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Sodium polystyrene sulfonate is not absorbed systemically following oral or rectal administration and maternal use is not expected to result in fetal ...
-
10 OVERDOSAGEOverdosage may result in electrolyte disturbances including hypokalemia, hypocalcemia, and hypomagnesemia. Appropriate measures should be taken to correct serum electrolytes (potassium, calcium ...
-
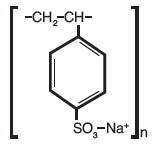
11 DESCRIPTIONSodium polystyrene sulfonate is a benzene, diethenyl-polymer, with ethenylbenzene, sulfonated, sodium salt and has the following structural formula: The drug is a cream to light brown finely ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sodium polystyrene sulfonate is a non-absorbed, cation exchange polymer that contains a sodium counterion. Sodium polystyrene sulfonate increases fecal potassium ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies have not been performed.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSodium polystyrene sulfonate is available as a cream to light brown, finely ground powder in jars of 1 pound (453.6 g), NDC 51293-831-97. Store at 25° C (77° F); excursions permitted to 15° ...
-
17 PATIENT COUNSELING INFORMATIONDrug Interactions - Advise patients who are taking other oral medication to separate the dosing of Sodium polystyrene sulfonate by at least 3 hours (before or after) [see Dosage and ...
-
SPL UNCLASSIFIED SECTIONRx Only - Manufactured for: ECI Pharmaceuticals, LLC - Fort Lauderdale, FL 33309 - Rev.: 02/2018
-
PRINCIPAL DISPLAY PANEL - 454 g Jar LabelNDC 51293-831-97 Rx Only - Sodium Polystyrene - Sulfonate, USP - Read Package Outsert - Average adult dose: 15 g (approximately 4 level Teaspoons) one to four times - daily in water. See ...
-
INGREDIENTS AND APPEARANCEProduct Information