Label: METHIMAZOLE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 51293-820-01, 51293-821-01 - Packager: ECI Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 27, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
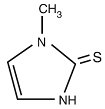
DESCRIPTIONMethimazole, USP (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from the drugs of the thiouracil series primarily because it ...
-
CLINICAL PHARMACOLOGYMethimazole inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and tri-iodothyronine that are ...
-
INDICATIONS AND USAGEMethimazole is indicated: In patients with Graves’ disease with hyperthyroidism or toxic multinodular goiter for whom surgery or radioactive iodine therapy is not an appropriate treatment option ...
-
CONTRAINDICATIONSMethimazole is contraindicated in the presence of hypersensitivity to the drug or any of the other product components.
-
WARNINGSCongenital Malformations - Methimazole readily crosses placental membranes and can cause fetal harm, particularly when administered in the first trimester of pregnancy. Rare instances of ...
-
PRECAUTIONSGeneral - Patients who receive methimazole should be under close surveillance and should be cautioned to report immediately any evidence of illness, particularly sore throat, skin eruptions ...
-
ADVERSE REACTIONSMajor adverse reactions (which occur with much less frequency than the minor adverse reactions) include inhibition of myelopoieses (agranulocytosis, granulocytopenia, thrombocytopenia, and ...
-
OVERDOSAGESigns and Symptoms - Symptoms may include nausea, vomiting, epigastric distress, headache, fever, joint pain, pruritus, and edema. Aplastic anemia (pancytopenia) or agranulocytosis may be ...
-
PRINCIPAL DISPLAY PANEL - Container Label - 5 mgNDC 51293-820-01 - ECI Pharmaceuticals - Methimazole - Tablets, USP - 5 mg - Rx Only - 100 Tablets
-
PRINCIPAL DISPLAY PANEL - Container Label - 10 mgNDC 51293-821-01 - ECI Pharmaceuticals - Methimazole - Tablets, USP - 10 mg - Rx Only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information