Label: CHOLESTYRAMINE powder, for suspension
- NDC Code(s): 51224-011-10, 51224-011-20
- Packager: TAGI Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
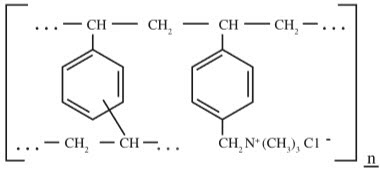
DESCRIPTIONCholestyramine for Oral Suspension, USP, the chloride salt of a basic anion exchange resin, a cholesterol lowering agent, is intended for oral administration. Cholestyramine resin is quite ...
-
CLINICAL PHARMACOLOGYCholesterol is probably the sole precursor of bile acids. During normal digestion, bile acids are secreted into the intestines. A major portion of the bile acids is absorbed from the intestinal ...
-
INDICATIONS AND USAGE1) Cholestyramine for Oral Suspension, USP is indicated as adjunctive therapy to diet for the reduction of elevated serum cholesterol in patients with primary hypercholesterolemia (elevated low ...
-
CONTRAINDICATIONSCholestyramine for Oral Suspension, USP is contraindicated in patients with complete biliary obstruction where bile is not secreted into the intestine and in those individuals who have shown ...
-
PRECAUTIONSGeneral - Chronic use of cholestyramine resin may be associated with increased bleeding tendency due to hypoprothrombinemia associated with Vitamin K deficiency. This will usually respond ...
-
ADVERSE REACTIONSThe most common adverse reaction is constipation. When used as a cholesterol-lowering agent predisposing factors for most complaints of constipation are high dose and increased age (more than 60 ...
-
OVERDOSAGEOverdosage of cholestyramine resin has been reported in a patient taking 150% of the maximum recommended daily dosage for a period of several weeks. No ill effects were reported. Should an ...
-
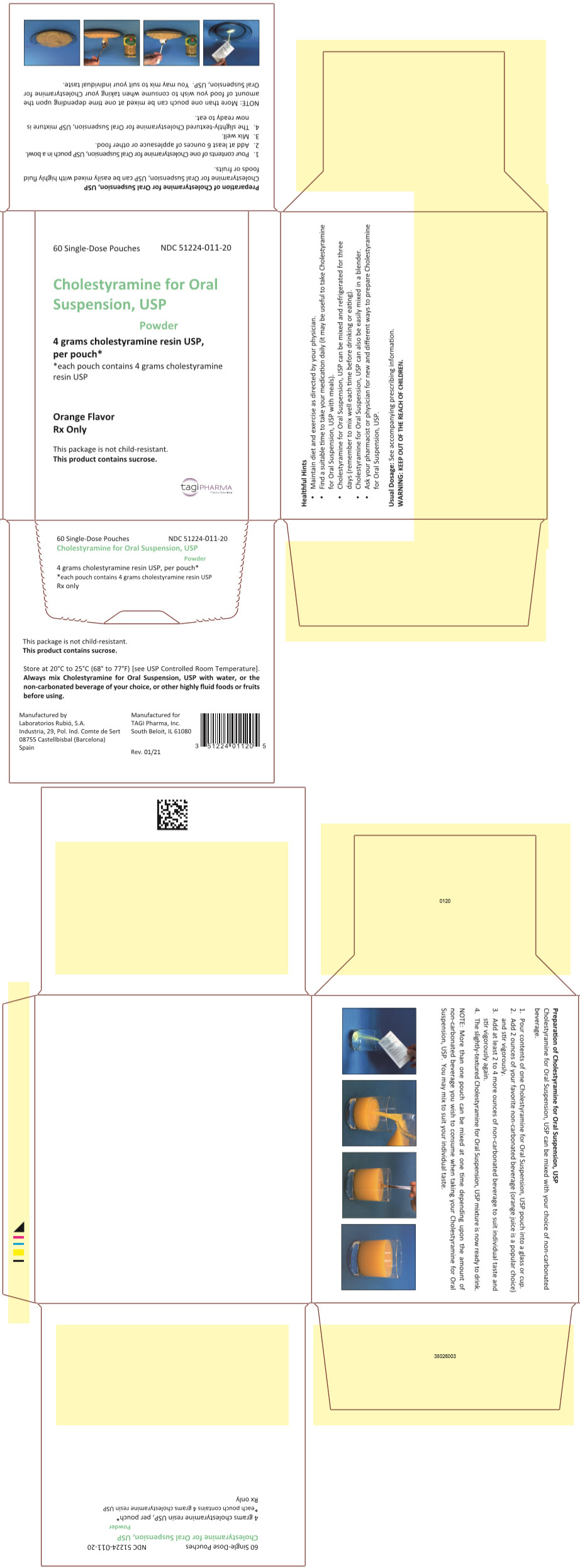
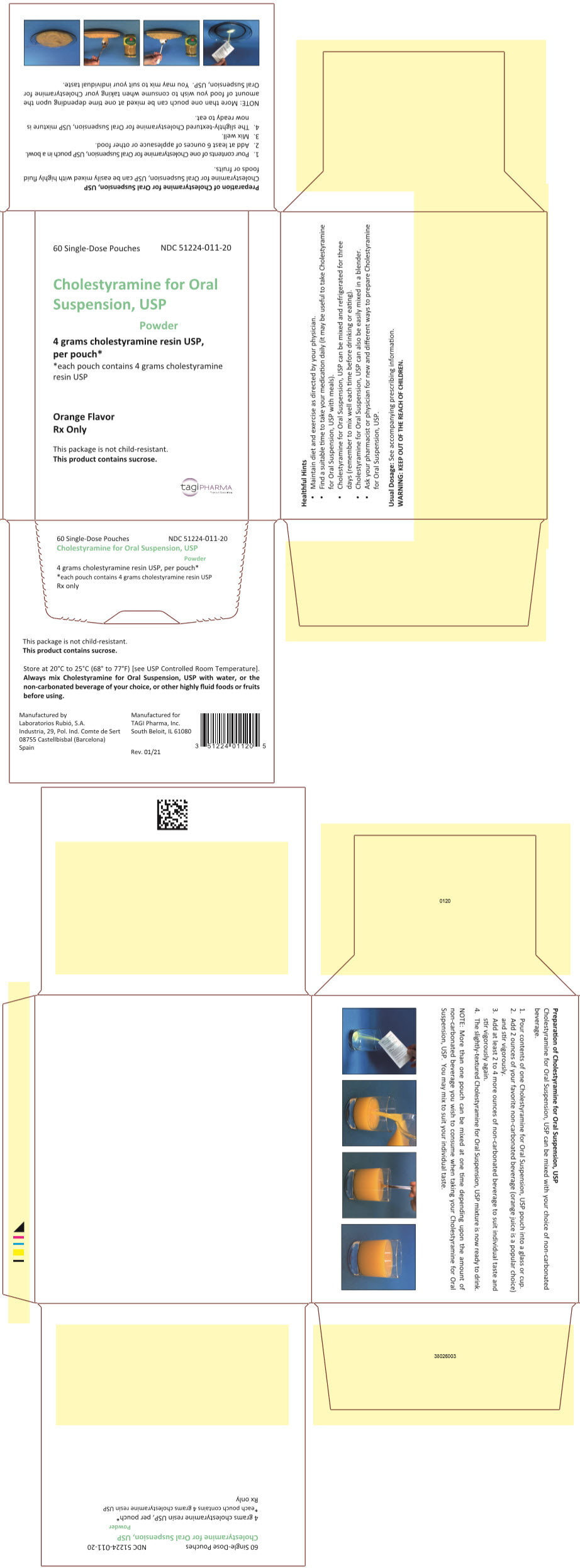
DOSAGE AND ADMINISTRATIONThe recommended starting adult dose for Cholestyramine for Oral Suspension, USP is 1 pouch or 1 level scoopful (8.3 grams of Cholestyramine for Oral Suspension, USP contains 4 grams of anhydrous ...
-
HOW SUPPLIEDCholestyramine for Oral Suspension, USP orange flavor is available in cartons of sixty 8.3 gram pouches and in a can containing 348.6 grams. 8.3 grams of Cholestyramine for Oral Suspension, USP ...
-
REFERENCES1. The Lipid Research Clinics Coronary Primary Prevention Trial Results: (I) Reduction in Incidence of Coronary Heart Disease; (II) The Relationship of Reduction in Incidence of Coronary Heart ...
-
SPL UNCLASSIFIED SECTIONTo report SUSPECTED ADVERSE REACTIONS, contact TAGI Pharma, Inc. at 1-844-668-3942 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Manufactured by - Laboratorios Rubió, S.A. Industria, 29, Pol ...
-
PRINCIPAL DISPLAY PANEL - 4 g Pouch Carton60 Single-Dose Pouches - NDC 51224-011-20 - Cholestyramine for Oral - Suspension, USP - Powder - 4 grams cholestyramine resin USP, per pouch* *each pouch contains 4 grams cholestyramine - resin USP - Orange ...
-
INGREDIENTS AND APPEARANCEProduct Information