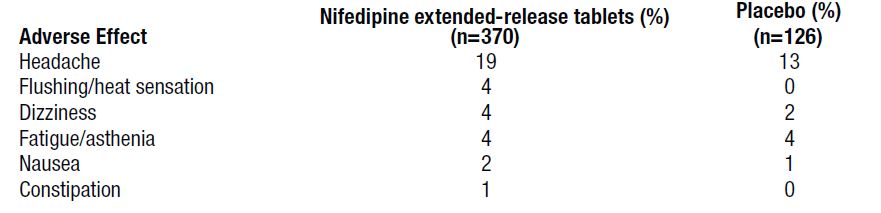Label: NIFEDIPINE tablet, extended release
- NDC Code(s): 50742-620-01, 50742-621-01, 50742-622-01
- Packager: Ingenus Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
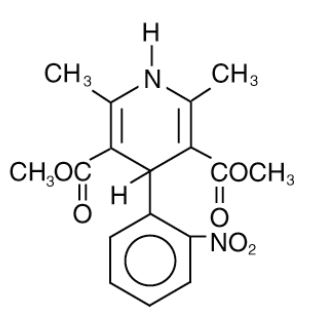
DESCRIPTIONNifedipine extended-release tablets, USP are an extended release tablet dosage form of the calcium channel blocker nifedipine. Nifedipine is 3,5-pyridinedicarboxylic acid ...
-
CLINICAL PHARMACOLOGYNifedipine is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) which inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac ...
-
INDICATIONS AND USAGENifedipine extended-release tablets, USP are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
-
CONTRAINDICATIONSConcomitant administration with strong P450 inducers, such as rifampin, are contraindicated since the efficacy of nifedipine tablets could be significantly reduced. (SeePRECAUTIONS, Drug ...
-
WARNINGSExcessive Hypotension - Although in most patients the hypotensive effect of nifedipine is modest and well tolerated, occasional patients have had excessive and poorly tolerated hypotension ...
-
PRECAUTIONSGeneral - Hypotension - Because nifedipine decreases peripheral vascular resistance, careful monitoring of blood pressure during the initial administration and titration of nifedipine ...
-
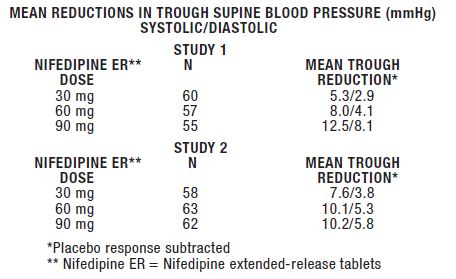
ADVERSE REACTIONSThe incidence of adverse events during treatment with nifedipine extendedrelease tablets in doses up to 90 mg daily were derived from multi-center placebo-controlled clinical trials in 370 ...
-
OVERDOSAGEExperience with nifedipine overdosage is limited. Symptoms associated with severe nifedipine overdosage include loss of consciousness, drop in blood pressure, heart rhythm disturbances, metabolic ...
-
DOSAGE AND ADMINISTRATIONDosage should be adjusted according to each patient's needs. It is recommended that nifedipine extended-release tablets, USP be administered orally once daily on an empty stomach. The nifedipine ...
-
HOW SUPPLIEDNifedipine extended-release tablets, USP are supplied as 30 mg, 60 mg, and 90 mg round film coated tablets. The different strengths can be identified as follows: Nifedipine extended-release ...
-
SPL UNCLASSIFIEDManufactured for: Ingenus Pharmaceuticals, LLC - Orlando, FL 32839-6408 - Manufactured by: Novast Laboratories Ltd. Nantong, China 226009 - Rx Only - I0086 - Iss. 03/2018 - Rev. C
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNifedipine Extended Release Tablets 30mg - 100ct Label - NDC 50742-620-01
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNifedipine Extended Release Tablets 60mg - 100ct Label - NDC 50742-621-01
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNifedipine Extended Release Tablets 90mg - 100ct Label - NDC 50742-622-01
-
INGREDIENTS AND APPEARANCEProduct Information