Label: SCOPOLAMINE patch, extended release
- NDC Code(s): 50742-505-04, 50742-505-10, 50742-505-24
- Packager: Ingenus Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SCOPOLAMINE TRANSDERMAL SYSTEM safely and effectively. See full prescribing information for SCOPOLAMINE TRANSDERMAL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEScopolamine transdermal system is indicated in adults for the prevention of: nausea and vomiting associated with motion sickness. post-operative nausea and vomiting (PONV) associated with ...
-
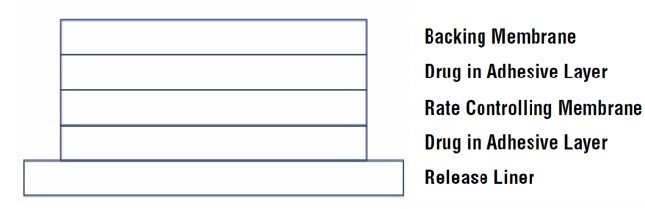
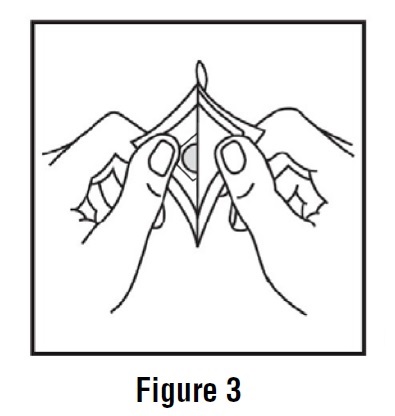
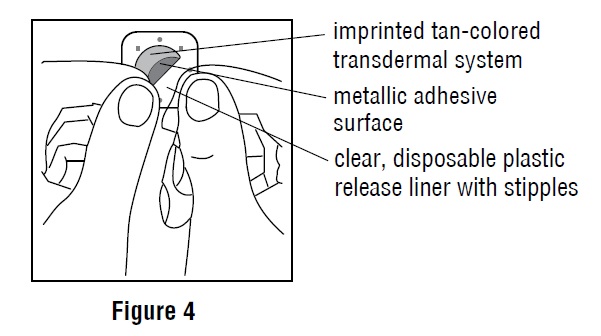
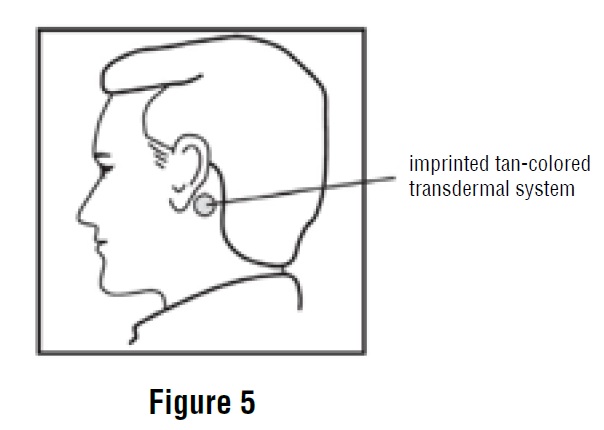
2 DOSAGE AND ADMINISTRATION2.1 Important Application and Removal Instructions - Each scopolamine transdermal system is formulated to deliver in vivo approximately 1 mg of scopolamine over 3 days. Only wear one transdermal ...
-
3 DOSAGE FORMS AND STRENGTHSTransdermal system: a circular, flat, tan-colored transdermal system imprinted with “Scopolamine 1 mg/3 days”
-
4 CONTRAINDICATIONSScopolamine transdermal system is contraindicated in patients with: angle closure glaucoma [see Warnings and Precautions (5.1)]. hypersensitivity to scopolamine or other belladonna alkaloids or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Acute Angle Closure Glaucoma - The mydriatic effect of scopolamine may cause an increase in intraocular pressure resulting in acute angle closure glaucoma. Monitor intraocular pressure in ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in labeling: Acute Angle Closure Glaucoma [see Warnings and Precautions (5.1)] Neuropsychiatric Adverse Reactions [see ...
-
7 DRUG INTERACTIONS7.1 Drugs Causing Central Nervous System (CNS) Adverse Reactions - The concurrent use of scopolamine transdermal system with other drugs that cause CNS adverse reactions of drowsiness, dizziness ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from observational studies and postmarketing reports with scopolamine use in pregnant women have not identified a drug associated risk of major ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance Class - Scopolamine transdermal system contains scopolamine, which is not a controlled substance. 9.3 Dependence - Termination of scopolamine transdermal system ...
-
10 OVERDOSAGEThe signs and symptoms of anticholinergic toxicity include: lethargy, somnolence, coma, confusion, agitation, hallucinations, convulsion, visual disturbance, dry flushed skin, dry mouth, decreased ...
-
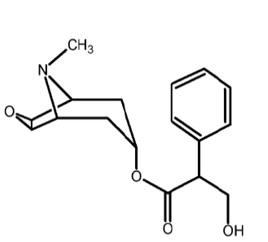
11 DESCRIPTIONScopolamine transdermal system is designed for continuous release of scopolamine following application to an area of intact skin on the head, behind the ear. Each system contains 1.5 mg of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Scopolamine, a belladonna alkaloid, is an anticholinergic. Scopolamine acts: i) as a competitive inhibitor at postganglionic muscarinic receptor sites of the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term studies in animals have been conducted to evaluate the carcinogenic potential of scopolamine. The mutagenic potential of ...
-
14 CLINICAL STUDIES14.1 Prevention of Motion Sickness - In 195 adult subjects of different racial origins who participated in clinical efficacy studies at sea or in a controlled motion environment, there was a 75 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGScopolamine transdermal system 1 mg/3 days is available as the following: Carton of 4 transdermal systems, packaged into individual foil pouches. NDC 50742-505-04 - Carton of 10 transdermal systems ...
-
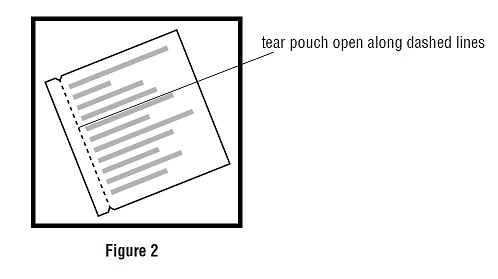
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Administration Instructions - Counsel patients on how to apply and remove the transdermal ...
-
MEDICATION GUIDEDispense with Medication Guide available at: www.ingenus.com/medguide/scopolamine-tds.pdf - MEDICATION GUIDE - Scopolamine (skoe-POL-a-meen) Transdermal System - Read this Medication ...
-
INSTRUCTIONS FOR USEScopolamine (skoe-POL-a-meen) Transdermal System - Read this Instructions for Use before you start using scopolamine transdermal system and each time you get a refill. There may be new ...
-
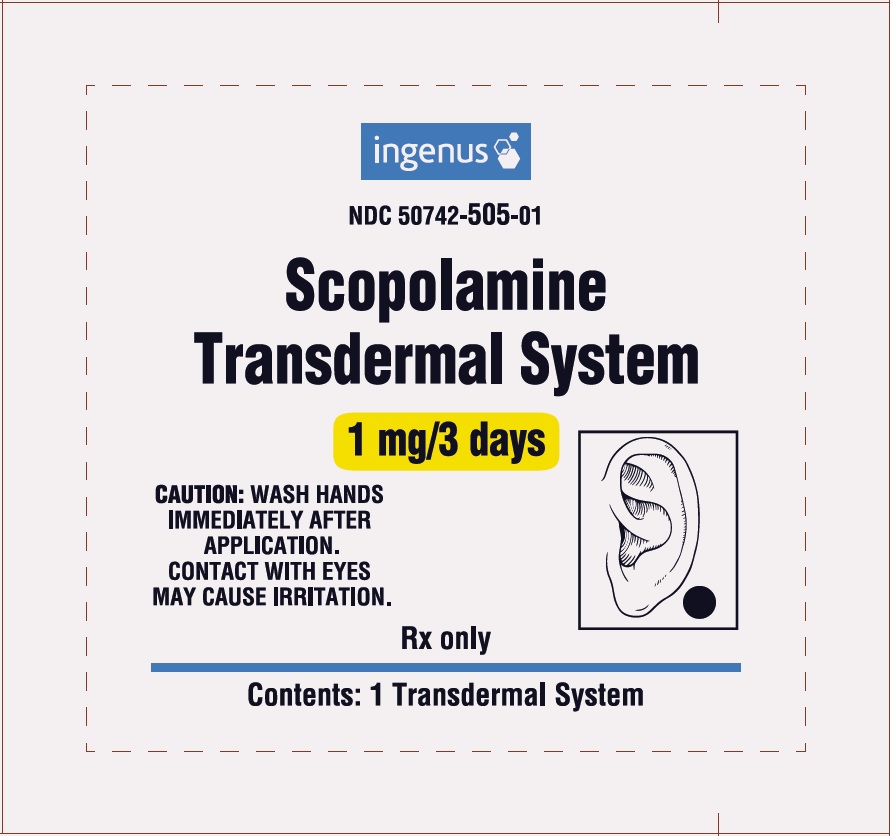
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – 1 Patch - ingenus - NDC 50742-505-01 - Scopolamine Transdermal System - 1 mg/3 days - CAUTION: WASH HANDS IMMEDIATELY AFTER APPLICATION. CONTACT WITH ...
-
PRINCIPAL DISPLAY PANELingenus - NDC 50742-505-04 - Scopolamine Transdermal System - 1 mg/3 days - Formulated delivery of approximately 1 mg over three days - MOTION SICKNESS & POST-OPERATIVE NAUSEA & VOMITING ...
-
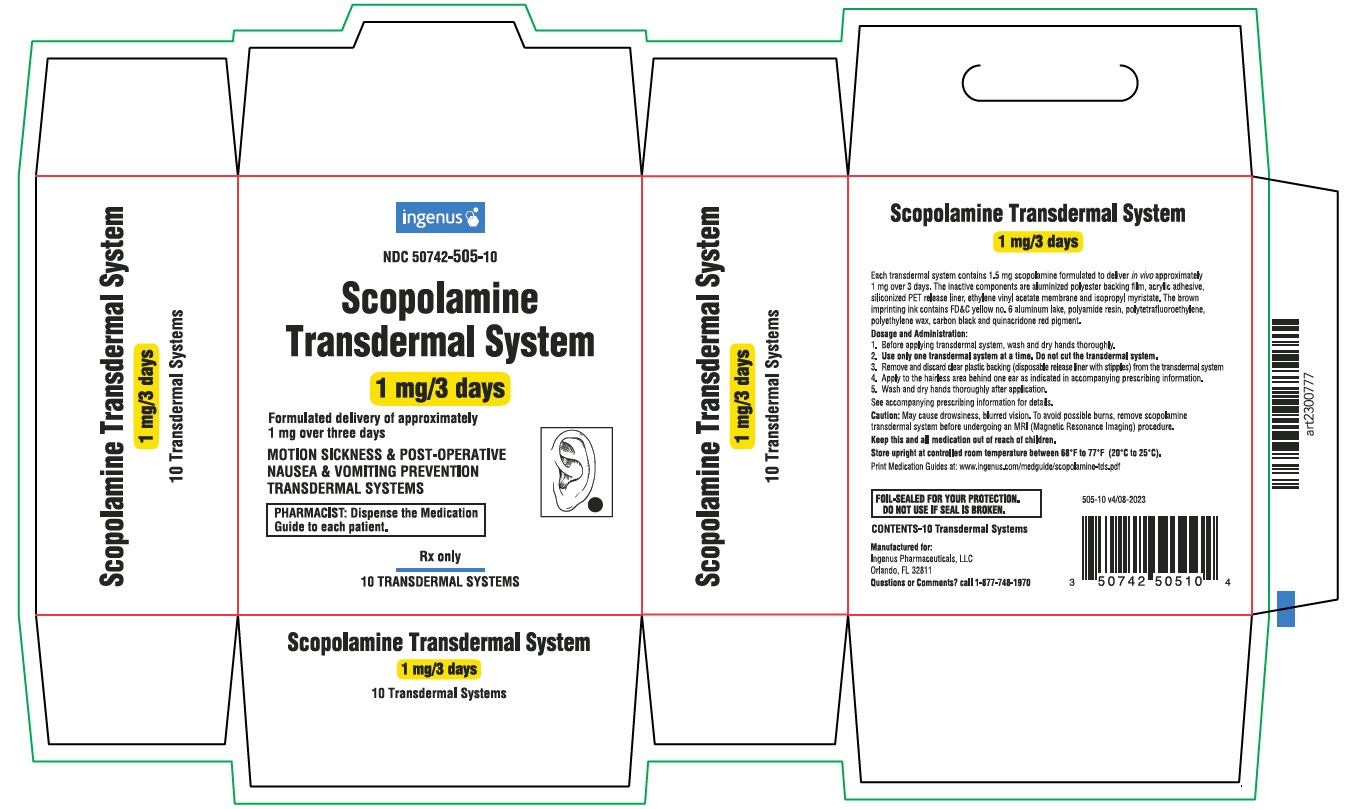
PRINCIPAL DISPLAY PANELingenus - NDC 50742-505-10 - Scopolamine Transdermal System - 1 mg/3 days - Formulated delivery of approximately 1 mg over three days - MOTION SICKNESS & POST-OPERATIVE NAUSEA & VOMITING ...
-
PRINCIPAL DISPLAY PANELingenus - NDC 50742-505-24 - Scopolamine Transdermal System - 1 mg/3 days - Formulated delivery of approximately 1 mg over three days - MOTION SICKNESS & POST-OPERATIVE NAUSEA & VOMITING ...
-
INGREDIENTS AND APPEARANCEProduct Information