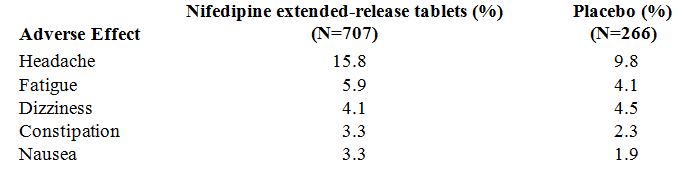Label: NIFEDIPINE tablet, extended release
- NDC Code(s): 50742-260-01, 50742-260-03, 50742-260-30, 50742-261-01, view more
- Packager: Ingenus Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
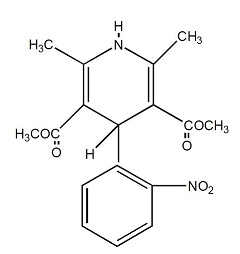
DESCRIPTIONNifedipine is a drug belonging to a class of pharmacological agents known as the calcium channel blockers. Nifedipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4 ...
-
CLINICAL PHARMACOLOGYNifedipine is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) and inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle. The ...
-
INDICATIONS AND USAGEI.Vasospastic Angina - Nifedipine extended-release tablets are indicated for the management of vasospastic angina confirmed by any of the following criteria: 1) classical pattern of angina at ...
-
CONTRAINDICATIONSKnown hypersensitivity reaction to nifedipine.
-
WARNINGSExcessive Hypotension - Although in most angina patients the hypotensive effect of nifedipine is modest and well tolerated, occasional patients have had excessive and poorly tolerated ...
-
PRECAUTIONSGeneral - Hypotension: Because nifedipine decreases peripheral vascular resistance, careful monitoring of blood pressure during the initial administration and titration of nifedipine is ...
-
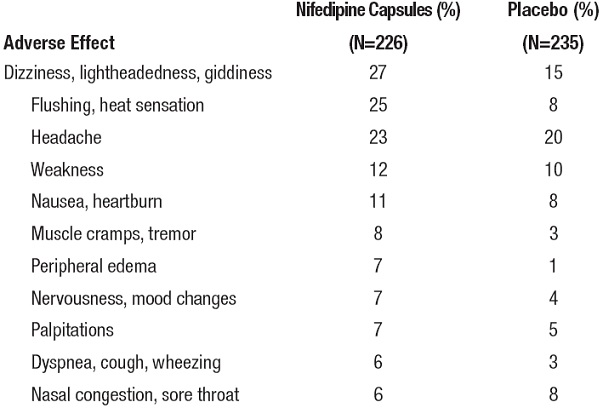
ADVERSE EXPERIENCESOver 1000 patients from both controlled and open trials with nifedipine extended-release tablets in hypertension and angina were included in the evaluation of adverse experiences. All side ...
-
OVERDOSAGEExperience with nifedipine overdosage is limited. Generally, overdosage with nifedipine leading to pronounced hypotension calls for active cardiovascular support, including monitoring of ...
-
DOSAGE AND ADMINISTRATIONDosage must be adjusted according to each patient's needs. Therapy for either hypertension or angina should be initiated with 30 or 60 mg once daily. Nifedipine extended-release tablets should be ...
-
HOW SUPPLIEDNifedipine extended-release tablets are supplied as 30 mg, 60 mg and 90 mg round, biconvex, film-coated tablets. The different strengths can be identified as follows: 30 mg: Yellow round biconvex ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Ingenus Pharmaceuticals, LLC - Orlando, FL 32839-6408 - Made in China - Rx Only - I0092 - Iss. 11/2022 - Rev. B
-
Nifedipine Extended Release Tablets 30mg - 100ct Label

-
Nifedipine Extended Release Tablets 60mg - 100ct Label

-
Nifedipine Extended Release Tablets 90mg - 100ct Label

-
INGREDIENTS AND APPEARANCEProduct Information