Label: CLONIDINE tablet, extended release
- NDC Code(s): 50742-247-05, 50742-247-30, 50742-247-60
- Packager: Ingenus Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLONIDINE HYDROCHLORIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for CLONIDINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEClonidine hydrochloride extended-release tablets are indicated for the treatment of attention deficit hyperactivity disorder (ADHD) as monotherapy and as adjunctive therapy to stimulant ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - Clonidine hydrochloride extended-release tablets is an extended-release tablet to be taken orally with or without food. Swallow tablets whole. Do not crush ...
-
3 DOSAGE FORMS AND STRENGTHSClonidine hydrochloride extended-release tablets are available as 0.1 mg strength extended-release tablets. The 0.1 mg tablets are white or off white, non-scored, round biconvex with debossing ...
-
4 CONTRAINDICATIONSClonidine hydrochloride extended-release tablets are contraindicated in patients with a history of a hypersensitivity reaction to clonidine. Reactions have included generalized rash ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension/Bradycardia - Treatment with clonidine hydrochloride extended-release tablets can cause dose-related decreases in blood pressure and heart rate [see Adverse Reactions (6.1)] ...
-
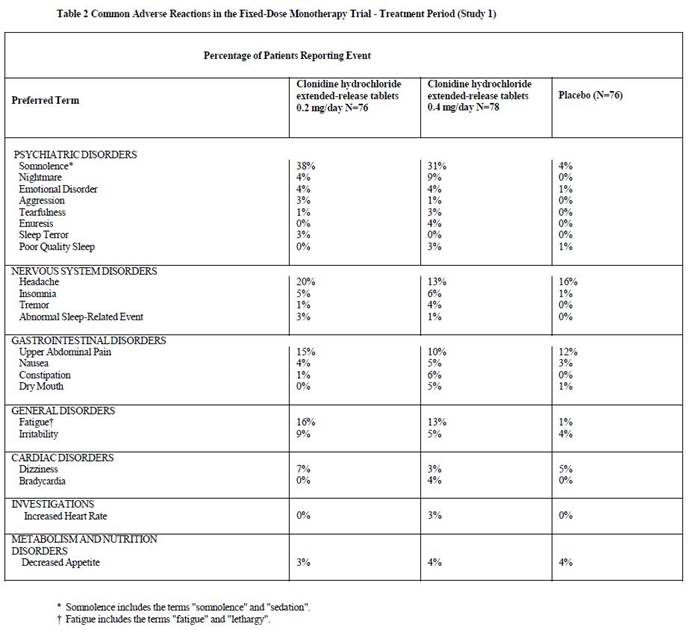
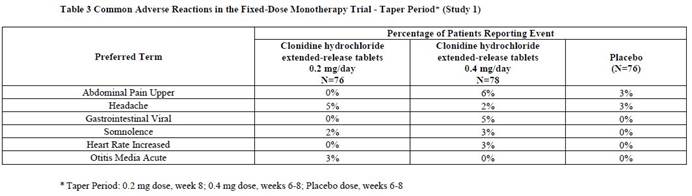
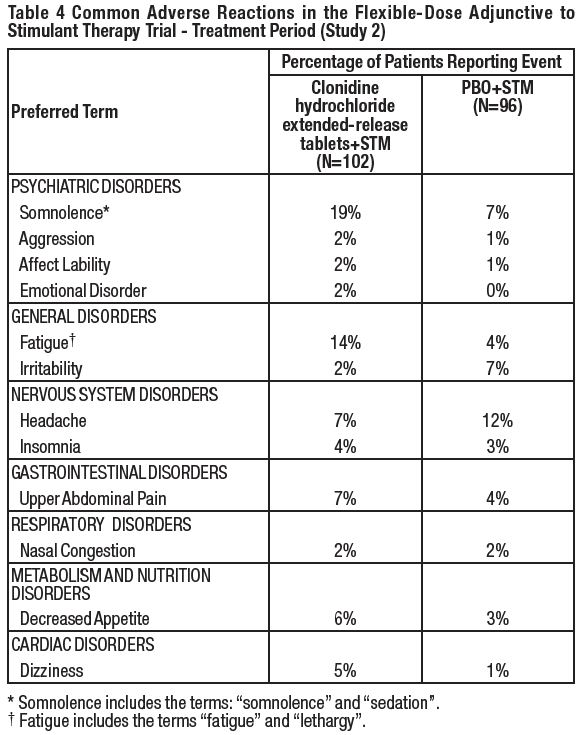
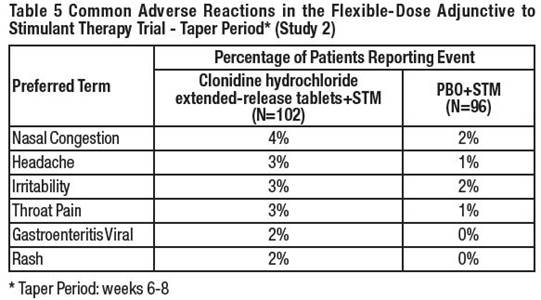
6 ADVERSE REACTIONSThe following serious adverse reactions are described in greater detail elsewhere in labeling: Hypotension/bradycardia [see Warnings and Precautions (5.1)] Sedation and somnolence [see ...
-
7 DRUG INTERACTIONSThe following have been reported with other oral immediate release formulations of clonidine: Table 6 Clinically Important Drug Interactions - Concomitant Drug Name or Drug Class ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including clonidine hydrochloride ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Clonidine hydrochloride extended-release tablets is not a controlled substance and has no known potential for abuse or dependence.
-
10 OVERDOSAGESymptoms - Clonidine overdose: hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes ...
-
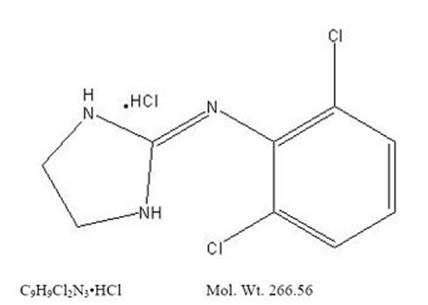
11 DESCRIPTIONClonidine hydrochloride extended-release is a centrally acting alpha2-adrenergic agonist available as 0.1 mg extended-release tablets for oral administration. Each 0.1 mg tablet is equivalent to ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clonidine stimulates alpha2-adrenergic receptors in the brain. Clonidine is not a central nervous system stimulant. The mechanism of action of clonidine in ADHD is not ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility - Carcinogenesis - Clonidine HCl was not carcinogenic when administered in the diet of rats (for up to 132 weeks) or mice (for up to ...
-
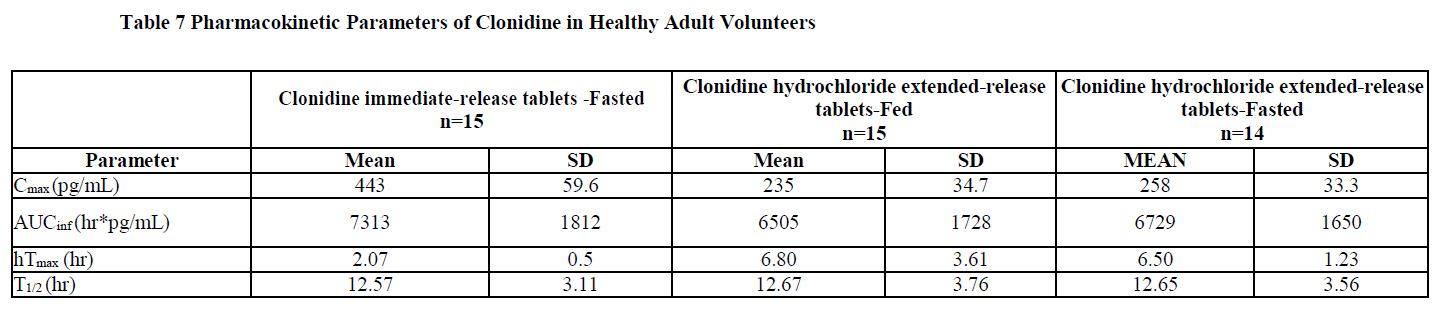
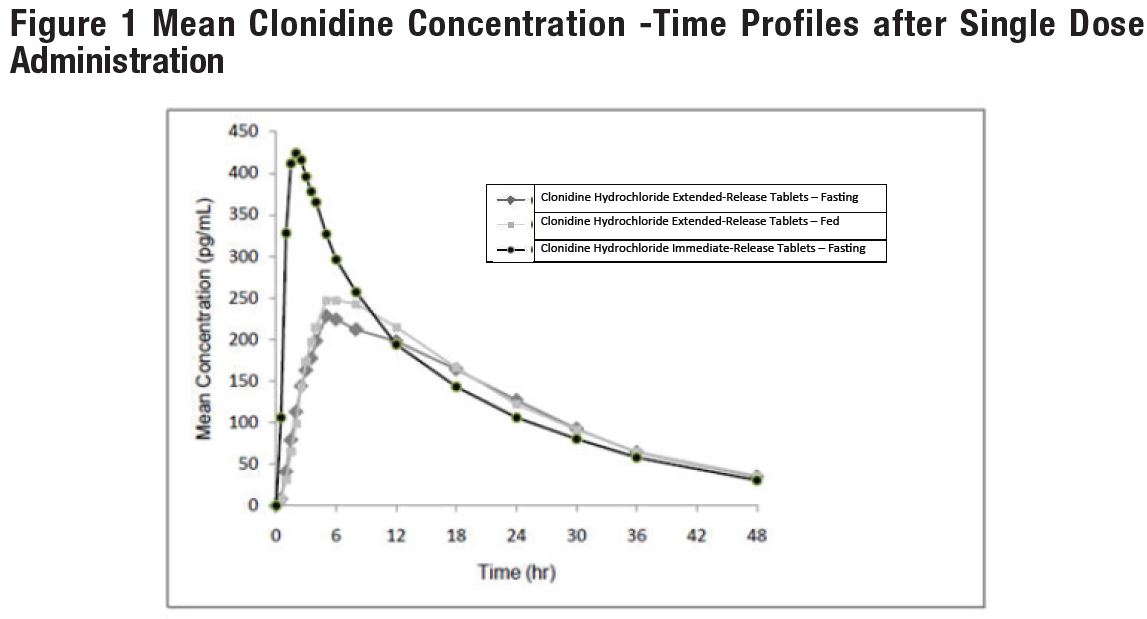
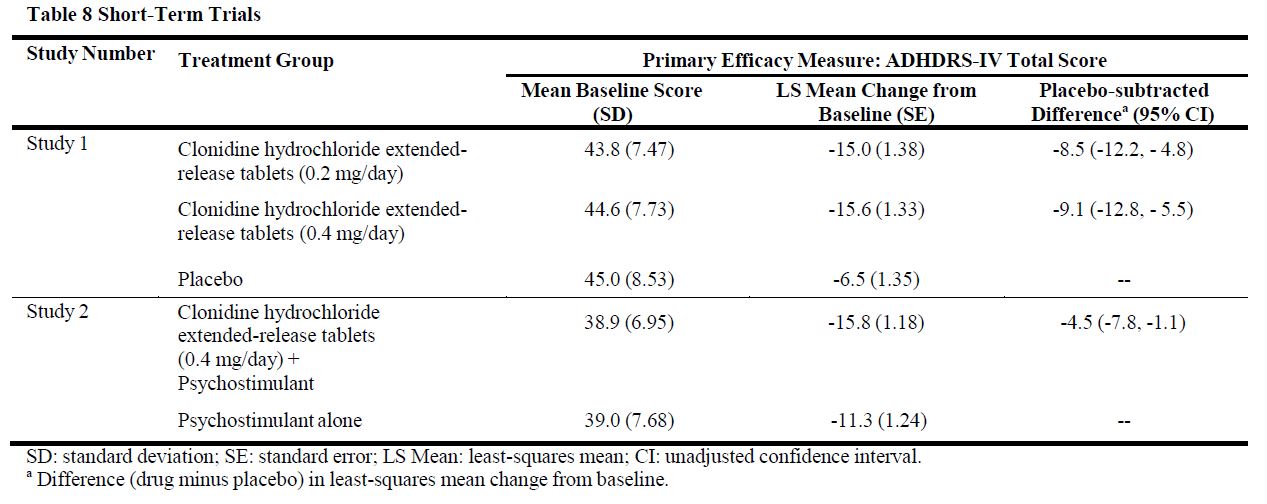
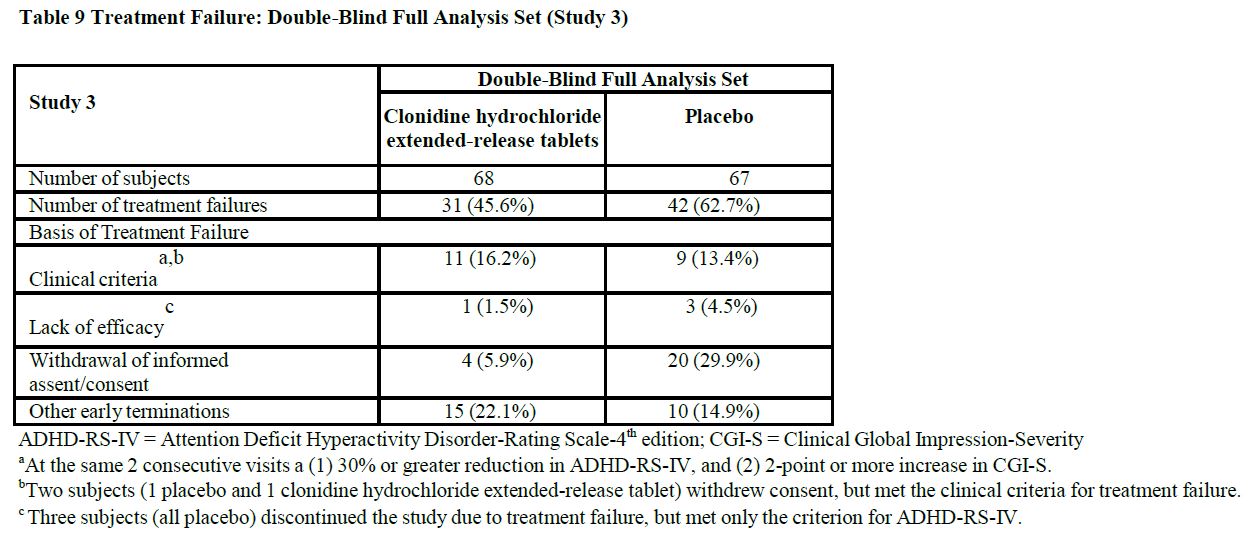
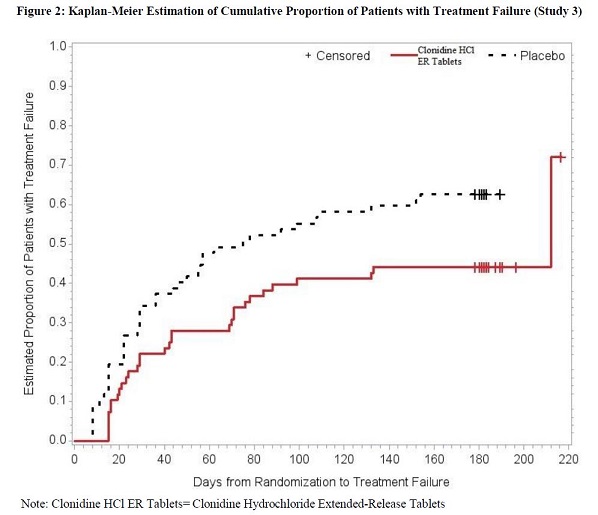
14 CLINICAL STUDIESEfficacy of clonidine hydrochloride extended-release tablets in the treatment of ADHD was established in children and adolescents (6 to 17 years) in: • One short-term, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGClonidine hydrochloride extended-release tablets are white or off white, non-scored, round biconvex with debossing ("NL 7") on one side. NDC 50742-247-30 – 0.1 mg round tablets supplied in ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved Patient Labeling (Patient Information) Dosage and Administration - Advise patients that clonidine hydrochloride extended-release tablets must be ...
-
Patient InformationClonidine Hydrochloride - (KLOE-ni-deen HYE-droe-KLOR-ide) Extended-Release Tablets - Read the Patient Information that comes with clonidine hydrochloride extended-release tablets before you ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information