Label: CYPROHEPTADINE HYDROCHLORIDE tablet
- NDC Code(s): 50742-190-01, 50742-190-10
- Packager: Ingenus Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 7, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
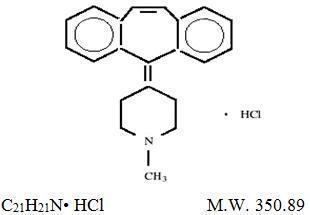
DESCRIPTIONCyproheptadine HCl USP, is an antihistaminic and antiserotonergic agent. Cyproheptadine hydrochloride USP is a white to slightly yellowish crystalline solid, with a molecular weight of 350.89 ...
-
CLINICAL PHARMACOLOGYCyproheptadine is a serotonin and histamine antagonist with anticholinergic and sedative effects. Antiserotonin and antihistamine drugs appear to compete with serotonin and histamine ...
-
INDICATIONS AND USAGE Perennial and seasonal allergic rhinitis - Vasomotor rhinitis - Allergic conjunctivitis due to inhalant allergens and foods - Mild, uncomplicated allergic skin manifestations of urticaria and ...
-
CONTRAINDICATIONS Newborn or Premature Infants - This drug should not be used in newborn or premature infants. Nursing Mothers - Because of the higher risk of antihistamines for infants generally and for ...
-
WARNINGS Pediatric Patients - Overdosage of antihistamines, particularly in infants and young children, may produce hallucinations, central nervous system depression, convulsions, respiratory and cardiac ...
-
PRECAUTIONS General - Cyproheptadine has an atropine-like action and, therefore, should be used with caution in patients with: History of bronchial asthma - Increased intraocular ...
-
ADVERSE REACTIONSTo report SUSPECTED ADVERSE REACTIONS, please call Ingenus Pharmaceuticals NJ, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch - Adverse reactions which have been reported ...
-
OVERDOSAGEAntihistamine overdosage reactions may vary from central nervous system depression to stimulation especially in pediatric patients. Also, atropine-like signs and symptoms (dry mouth; fixed ...
-
DOSAGE AND ADMINISTRATIONDOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT. Each tablet contains 4 mg of cyproheptadine hydrochloride. Pediatric Patients - Age 2 to 6 years - The ...
-
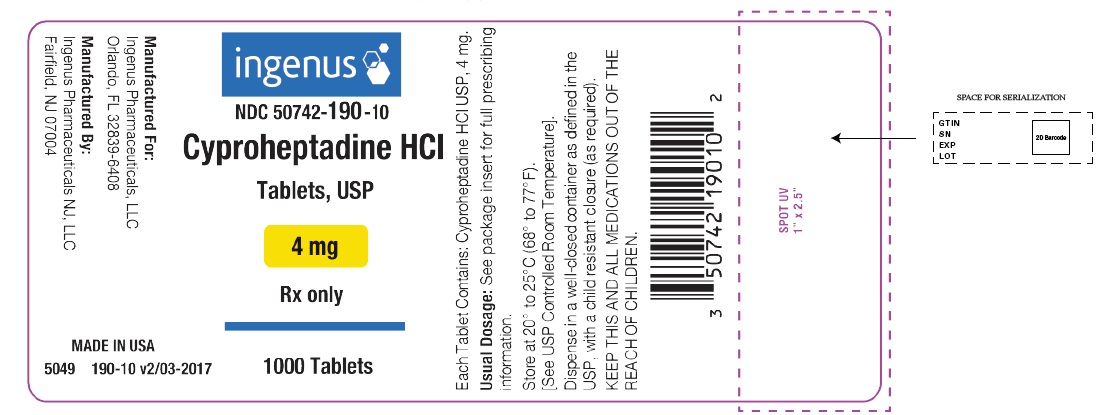
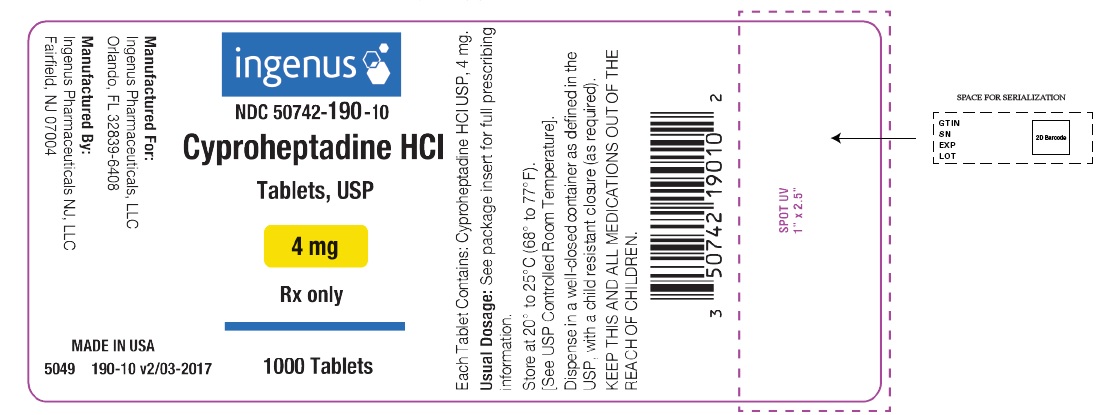
HOW SUPPLIEDCyproheptadine Hydrochloride Tablets USP, 4 mg are available as white to off- white, round, flat-faced, beveled-edged tablets, debossed “4” on left side, “9” on right side of the scoring on one ...
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – 4 mg, 100 Tablets Label - ingenus - NDC 50742-190-01 - Cyproheptadine HCI Tablets, USP - 4 mg - Rx only - 100 Tablets
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – 4 mg, 1000 Tablets Label - ingenus - NDC 50742-190-10 - Cyproheptadine HCI Tablets, USP - 4 mg - Rx only - 1000 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information