Label: CYCLOPHOSPHAMIDE injection, solution
- NDC Code(s): 50742-519-02, 50742-520-05, 50742-521-10
- Packager: Ingenus Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CYCLOPHOSPHAMIDE INJECTION safely and effectively. See full prescribing information for CYCLOPHOSPHAMIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Malignant Diseases - Cyclophosphamide is indicated for the treatment of: malignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkin's disease, lymphocytic lymphoma ...
-
2 DOSAGE AND ADMINISTRATIONDuring or immediately after the administration, adequate amounts of fluid should be ingested or infused to force diuresis in order to reduce the risk of urinary tract toxicity. Therefore ...
-
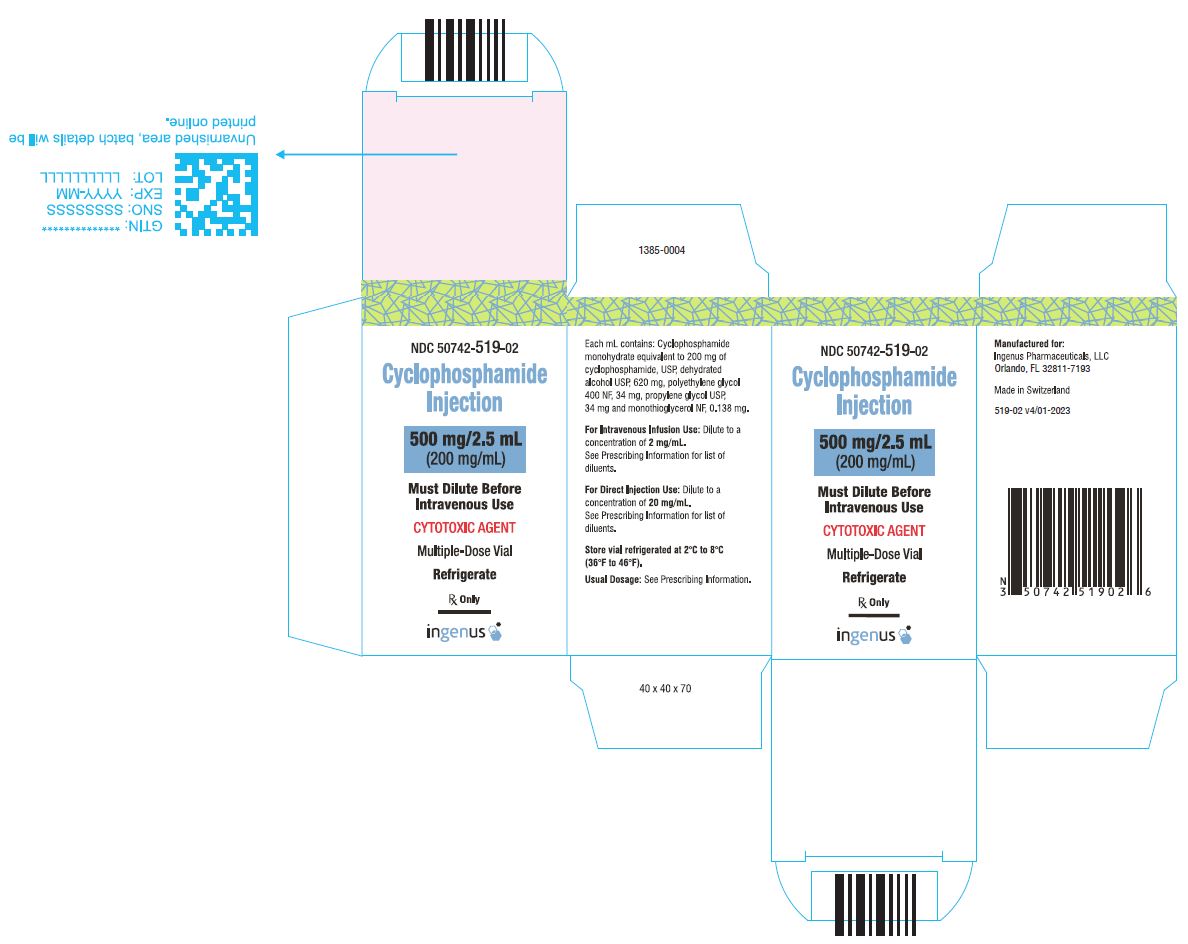
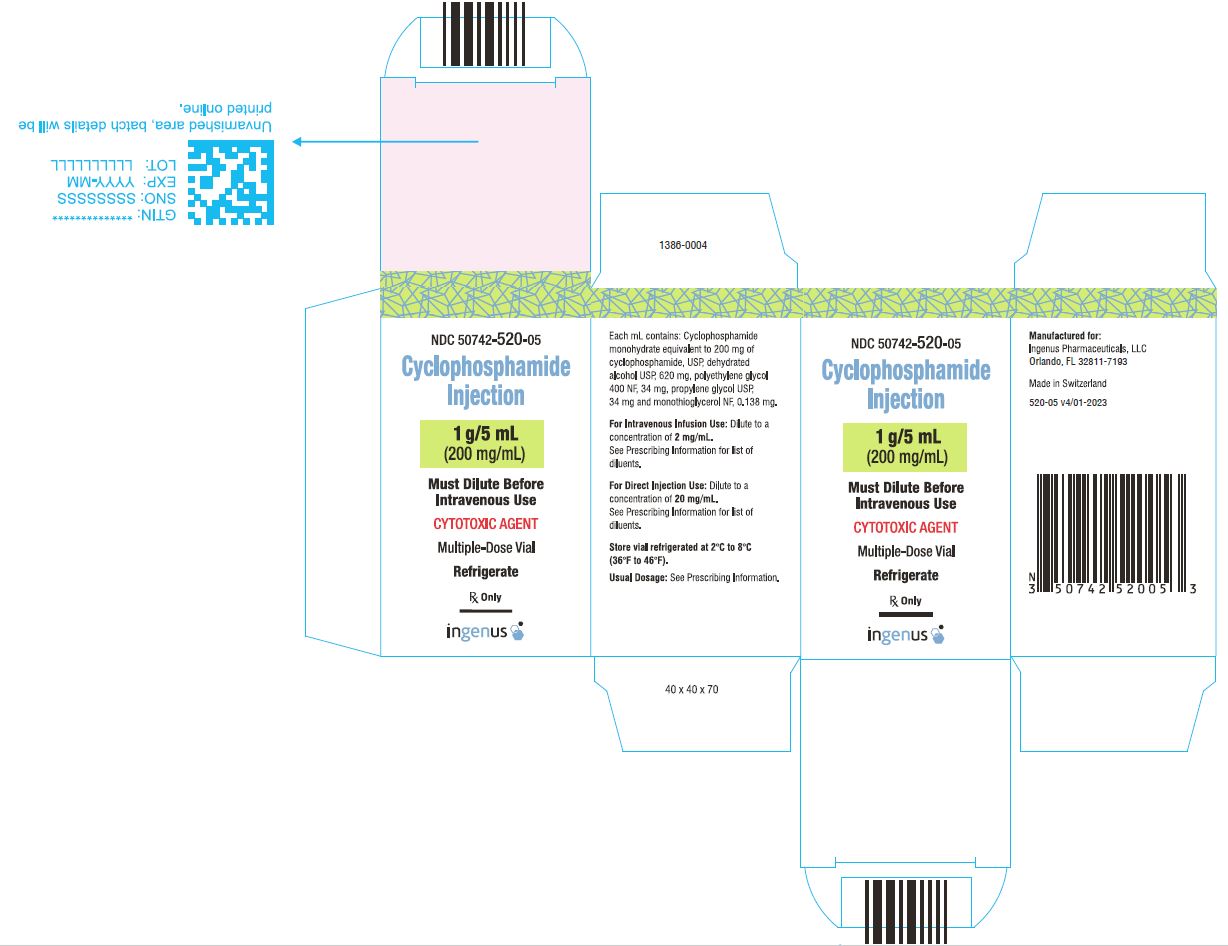
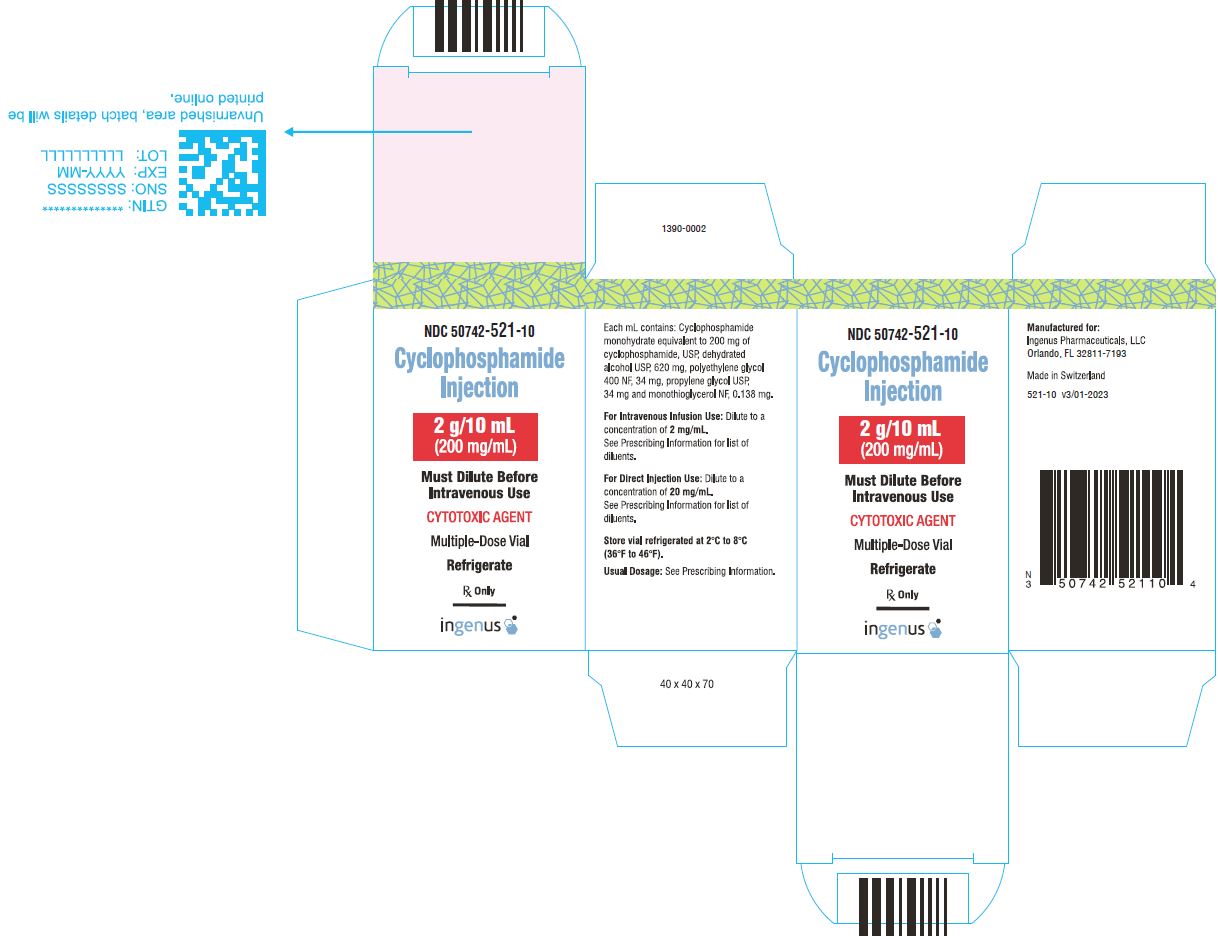
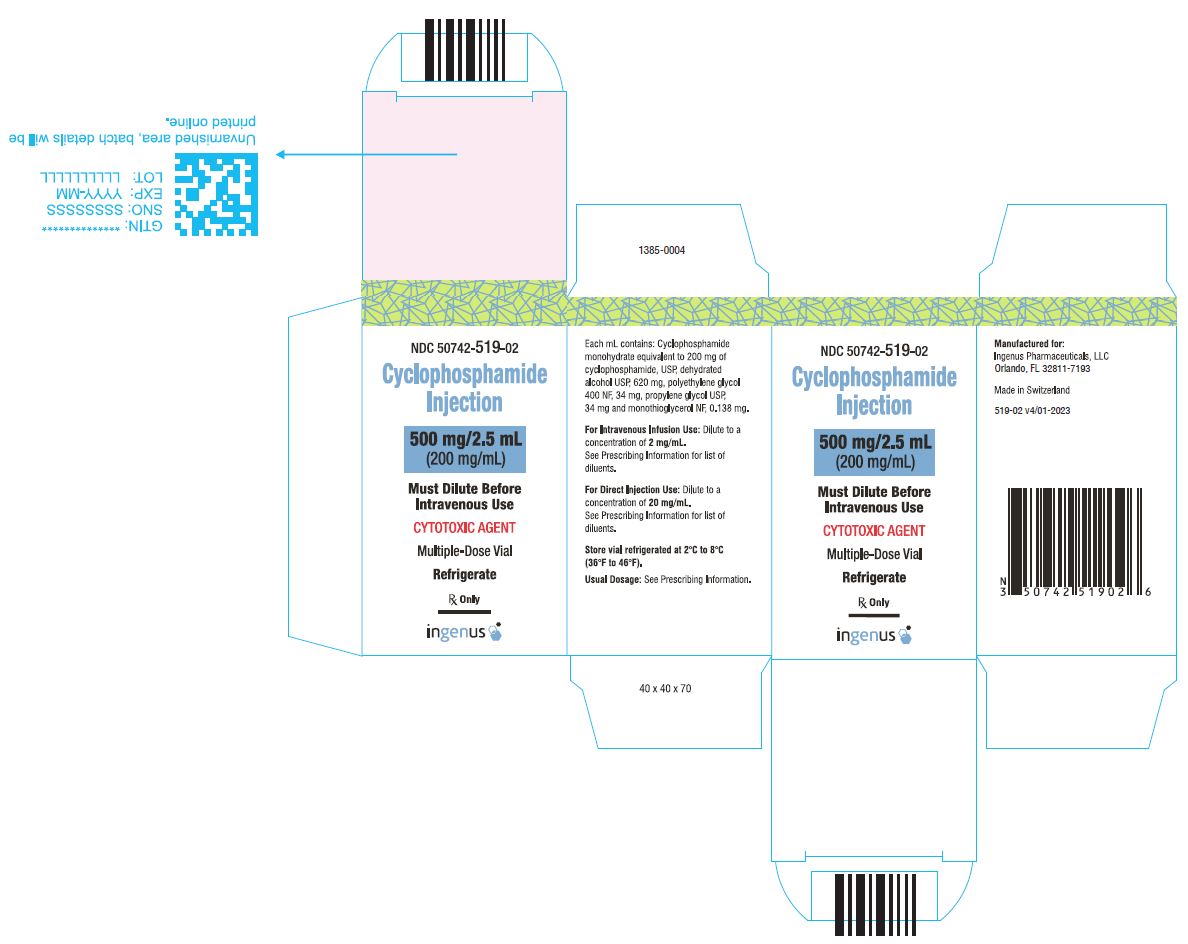
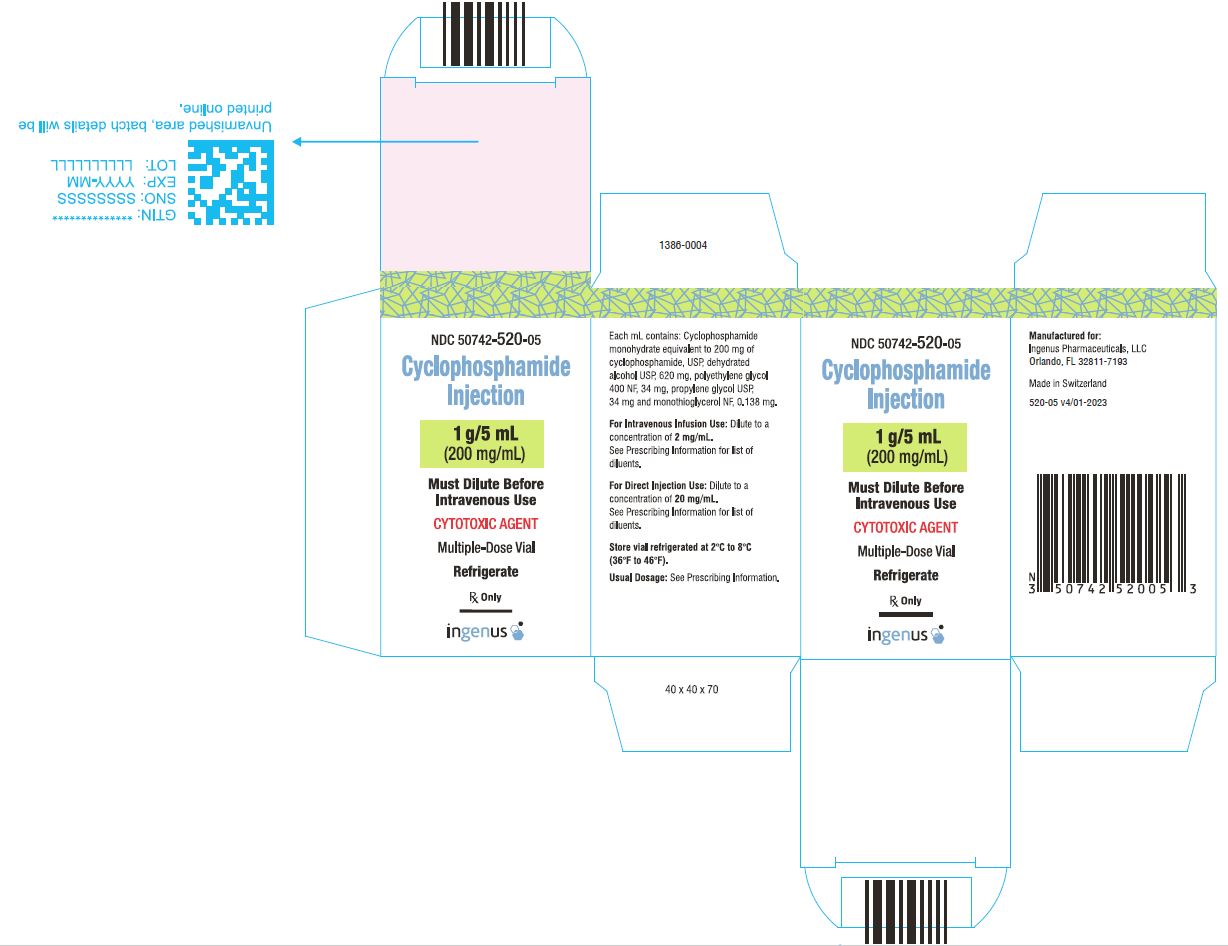
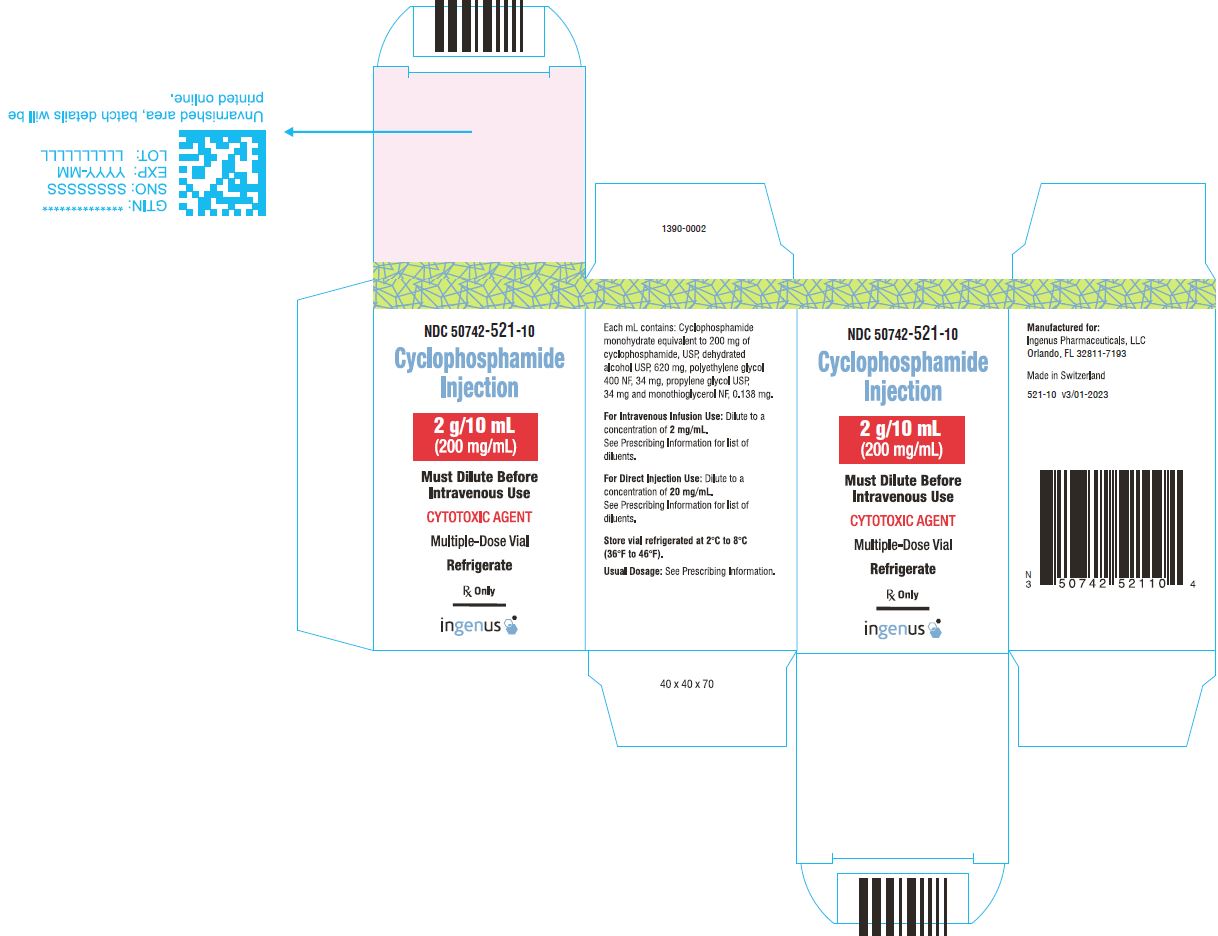
3 DOSAGE FORMS AND STRENGTHSCyclophosphamide Injection is a 200 mg/mL sterile clear colorless ready to dilute solution in a multiple-dose vial available in the following presentations: 500 mg/2.5 mL - 1 g/5 mL - 2 g/10 ...
-
4 CONTRAINDICATIONSHypersensitivity - Cyclophosphamide is contraindicated in patients who have a history of severe hypersensitivity reactions to it, any of its metabolites, or to other components of the product ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression, Immunosuppression, Bone Marrow Failure and Infections - Cyclophosphamide can cause myelosuppression (leukopenia, neutropenia, thrombocytopenia and anemia), bone marrow ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling. Hypersensitivity [see Contraindications (4)] Myelosuppression, Immunosuppression, Bone Marrow ...
-
7 DRUG INTERACTIONSCyclophosphamide is a pro-drug that is activated by cytochrome P450s [see Clinical Pharmacology (12.3)]. An increase of the concentration of cytotoxic metabolites may occur with: Protease ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and published reports of effects in pregnant patients or animals, Cyclophosphamide Injection can cause fetal harm when administered ...
-
10 OVERDOSAGENo specific antidote for cyclophosphamide is known. Overdosage should be managed with supportive measures, including appropriate treatment for any concurrent infection, myelosuppression, or ...
-
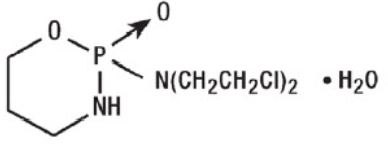
11 DESCRIPTIONCyclophosphamide is a synthetic antineoplastic drug chemically related to the nitrogen mustards. The chemical name for cyclophosphamide is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action is thought to involve cross-linking of tumor cell DNA. 12.2 Pharmacodynamics - Cyclophosphamide is biotransformed principally in the liver ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Cyclophosphamide administered by different routes, including intravenous, subcutaneous or intraperitoneal injection, or in drinking ...
-
15 REFERENCES1. OSHA Hazardous drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCyclophosphamide Injection is a 200 mg/mL sterile clear colorless solution ready-to-dilute solution containing cyclophosphamide, USP. Cyclophosphamide Injection - NDC Number - Strength ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient of the following: Myelosuppression, Immunosuppression, and Infections - Inform patients of the possibility of myelosuppression, immunosuppression, and infections. Explain the ...
-
PRINCIPAL DISPLAY PANELCyclophosphamide Injection, 500 mg/2.5 mL Vial Label - Cyclophosphamide Injection, 500 mg/2.5 mL Carton Label - Cyclophosphamide Injection, 1 g/5 mL vial label - Cyclophosphamide Injection, 1 g/5 ...
-
INGREDIENTS AND APPEARANCEProduct Information