Label: LOTEPREDNOL ETABONATE suspension/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 50383-265-05, 50383-265-10, 50383-265-15 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Sterile Ophthalmic Suspension Rev.265:00 05/17
-
DESCRIPTION
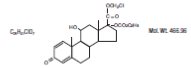
Loteprednol Etabonate Ophthalmic Suspension contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder. Loteprednol ...
-
CLINICAL PHARMACOLOGY Corticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte ...
-
INDICATIONS AND USAGE Loteprednol etabonate is indicated for the treatment of steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe such as ...
-
CONTRAINDICATIONS Loteprednol etabonate, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic ...
-
WARNINGS Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision, and in posterior subcapsular cataract formation. Steroids ...
-
PRECAUTIONS General - For ophthalmic use only. The initial prescription and renewal of the medication order beyond 14 days should be made by a physician only after examination of the patient with the aid of ...
-
ADVERSE REACTIONS To report SUSPECTED ADVERSE REACTIONS, contact Hi-Tech Pharmacal Co., Inc. at 1-800-262-9010 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Reactions associated with ophthalmic steroids include ...
-
DOSAGE AND ADMINISTRATION SHAKE VIGOROUSLY BEFORE USING. Steroid Responsive Disease Treatment: Apply one to two drops of loteprednol etabonate into the conjunctival sac of the affected eye four times daily. During the ...
-
HOW SUPPLIED Loteprednol Etabonate Ophthalmic Suspension, 0.5% is supplied in a plastic bottle with a controlled drop tip and a pink cap in the following sizes: 5 mL - NDC 50383-265-05 - 10 mL - NDC ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL AKORN - NDC 50383-265-05 - Loteprednol Etabonate Ophthalmic Suspension, 0.5% Rx only - 5 mL
-
INGREDIENTS AND APPEARANCEProduct Information