Label: LEVOCARNITINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 50383-172-90 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
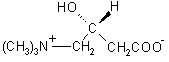
DESCRIPTIONLevocarnitine is a carrier molecule in the transport of long-chain fatty acids across the inner mitochondrial membrane. The chemical name of levocarnitine is ...
-
CLINICAL PHARMACOLOGYLevocarnitine is a naturally occurring substance required in mammalian energy metabolism. It has been shown to facilitate long-chain fatty acid entry into cellular mitochondria, thereby delivering ...
-
PHARMACOKINETICSIn a relative bioavailability study in 15 healthy adult male volunteers, levocarnitine tablets were found to be bio-equivalent to levocarnitine oral solution. Following 4 days of dosing with 6 ...
-
METABOLISM AND EXCRETIONIn a pharmacokinetic study where five normal adult male volunteers received an oral dose of [3H-methyl]-L-carnitine following 15 days of a high carnitine diet and additional carnitine supplement ...
-
INDICATIONS AND USAGELevocarnitine is indicated in the treatment of primary systemic carnitine deficiency. In the reported cases, the clinical presentation consisted of recurrent episodes of Reye-like encephalopathy ...
-
CONTRAINDICATIONSNone known.
-
WARNINGSHypersensitivity Reactions - Serious hypersensitivity reactions, including rash, urticaria, and facial edema have been reported with oral levocarnitine. Other serious hypersensitivity ...
-
PRECAUTIONSGeneral - The safety and efficacy of oral levocarnitine has not been evaluated in patients with renal insufficiency. Chronic administration of high doses of oral levocarnitine in patients with ...
-
ADVERSE REACTIONSThe following adverse reactions associated with the use of oral formulations of levocarnitine were identified in clinical trials or postmarketing reports. Because these reactions were reported ...
-
OVERDOSAGEThere have been no reports of toxicity from levocarnitine overdosage. Levocarnitine is easily removed from plasma by dialysis. The intravenous LD50 of levocarnitine in rats is 5.4 g/kg and the ...
-
DOSAGE AND ADMINISTRATIONLevocarnitine Tablets USP - Adults: The recommended oral dosage for adults is 990 mg two or three times a day using the 330 mg tablets, depending on clinical response. Infants and children: The ...
-
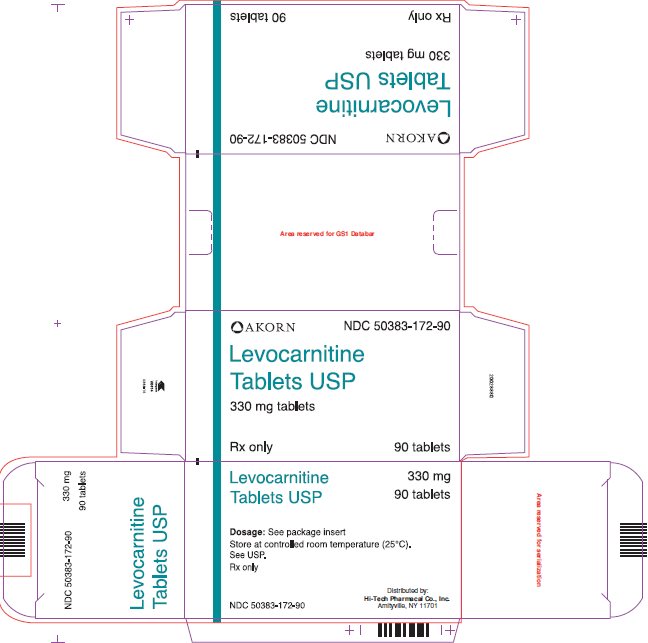
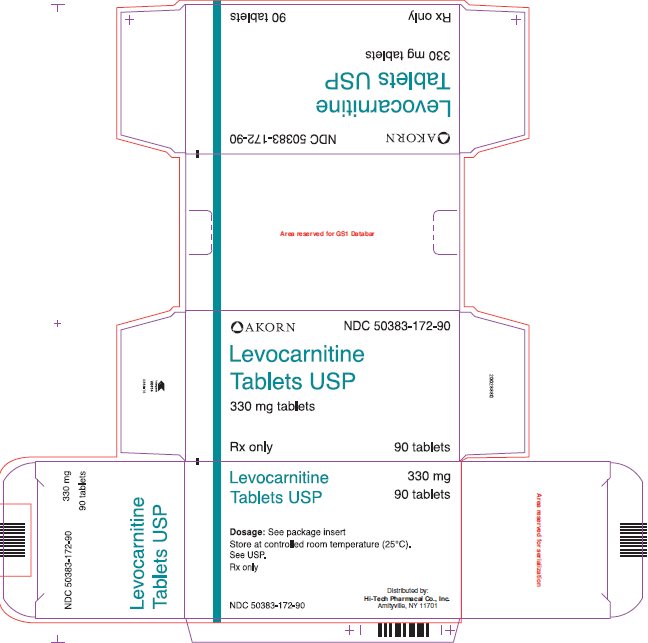
HOW SUPPLIEDLevocarnitine Tablets USP are supplied as 330 mg tablets embossed with "LC 77" in individual blisters, packaged in boxes of 90 (NDC 50383-172-90). Store at controlled room temperature (25°C). See ...
-
REFERENCES1. Bohmer, T., Rydning, A. and Solberg, H.E. 1974. Carnitine levels in human serum in health and disease. Clin. Chim. Acta 57:55-61. 2. Brooks, H., Goldberg, L., Holland, R. et al. 1977 ...
-
Package/Label Display Panel AKORN NDC 50383-172-90 - Levocarnitine Tablets USP - 330 mg tablets - Rx only 90 tablets
-
INGREDIENTS AND APPEARANCEProduct Information