IMPORTANT PATIENT INFORMATION AND INSTRUCTIONS.
Rev. 06/22
-
READ BEFORE USE.
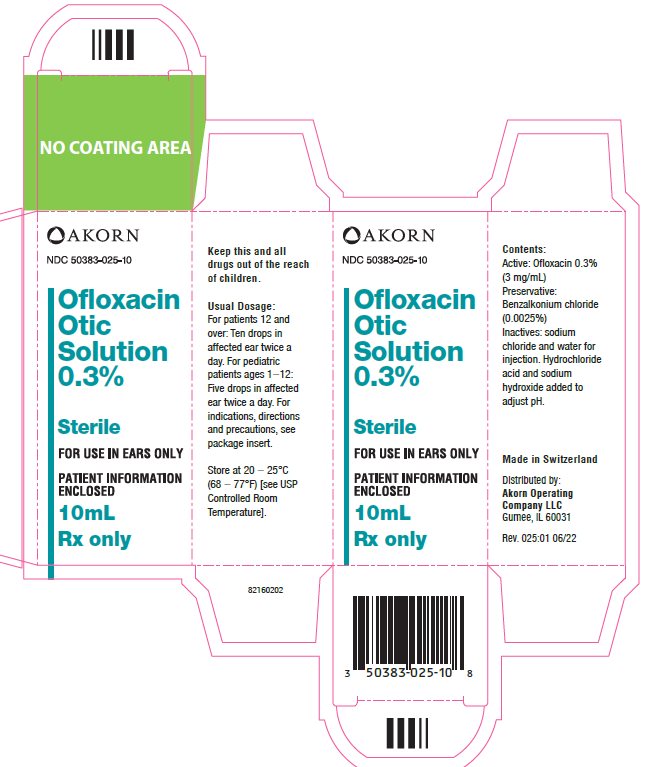
What is Ofloxacin Otic Solution?
Ofloxacin Otic Solution is an antibiotic in a sterile solution used to treat ...
IMPORTANT PATIENT INFORMATION AND INSTRUCTIONS.
Rev. 06/22
READ BEFORE USE.
What is Ofloxacin Otic Solution?
Ofloxacin Otic Solution is an antibiotic in a sterile solution used to treat ear infections caused by certain bacteria found in:
- •
- patients (12 years and older) who have middle ear infection and have a hole in the eardrum
- •
- pediatric patients (between 1 and 12 years of age) who have a middle ear infection and have a tube in the eardrum
- •
- patients (6 months and older) who have an infection in the ear canal
Middle Ear Infection: A middle ear infection is a bacterial infection behind the eardrum. People with a hole or a tube in the eardrum may notice a discharge (fluid draining) in the ear canal.
Ear Canal Infection: An ear canal infection (also known as “Swimmer’s Ear”) is a bacterial infection of the ear canal. The ear canal and the outer part of the ear may swell, turn red, and be painful. Also, a fluid discharge may appear in the ear canal.
Who should NOT use Ofloxacin Otic Solution?
- •
- Do not use this product if allergic to ofloxacin or to other quinolone antibiotics.
- •
- Do not give this product to pediatric patients who:
- •
- have an ear canal infection and are less than 6 months of age because no data were collected from this population
- •
- have a middle ear infection and have a tube in the eardrum and are less than one year of age because no data were collected from this population
- •
- have a middle ear infection and have a hole in the eardrum and are less than 12 years of age because no data were collected from this population
How should Ofloxacin Otic Solution be given?
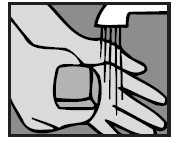
1. Wash hands
The person giving Ofloxacin Otic Solution should wash his/her hands with soap and water.
2. Clean ear & warm bottle
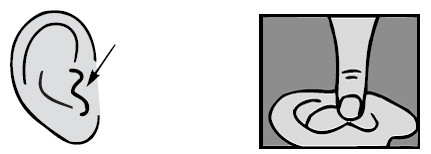
Gently clean any discharge that can be removed easily from the outer ear. DO NOT INSERT ANY OBJECT OR SWAB INTO THE EAR CANAL.
Hold the bottle of Ofloxacin Otic Solution in the hand for one or two minutes to warm the solution.
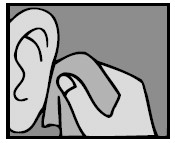
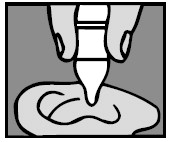
3. Add drops
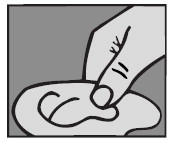
For a Middle Ear Infection: The person receiving Ofloxacin Otic Solution should lie on his/her side with the infected ear up. Patients (12 and older) should have 10 drops of Ofloxacin Otic Solution put into the infected ear. Pediatric patients under 12 should have 5 drops put into the infected ear. The tip of the bottle should not touch the fingers or the ear or any other surfaces.
For an Ear Canal Infection (“Swimmer’s Ear”): The person receiving Ofloxacin Otic Solution should lie on his/her side with the infected ear up. Patients (13 and older) should have 10 drops of Ofloxacin Otic Solution put into the infected ear. Pediatric patients under 13 should have 5 drops put into the infected ear. The tip of the bottle should not touch the fingers or the ear or any other surfaces.
BE SURE TO FOLLOW THE INSTRUCTIONS BELOW FOR THE PATIENT’S SPECIFIC EAR INFECTION.
4. Press ear or pull ear
For a Middle Ear Infection: While the person receiving Ofloxacin Otic Solution lies on his/her side, the person giving the drops should gently press the TRAGUS (see diagram) 4 times in a pumping motion. This will allow the drops to pass through the hole or tube in the eardrum and into the middle ear.
For an Ear Canal Infection (“Swimmer’s Ear”): While the person receiving the drops lies on his/her side, the person giving the drops should gently pull the outer ear upward and backward. This will allow the ear drops to flow down into the ear canal.
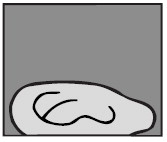
5. Stay on side
The person who received the ear drops should remain on his/her side for at least 5 minutes.
Repeat steps 2-5 for the other ear if both ears are infected.
How often should Ofloxacin Otic Solution be given?
In patients with an Ear Canal Infection (“Swimmer’s Ear”), Ofloxacin Otic Solution ear drops should be given once daily at about the same time each day (for example, 8 AM or 8 PM) in each infected ear unless the doctor has instructed otherwise.
In patients with a Middle Ear Infection, Ofloxacin Otic Solution ear drops should be given 2 times each day (about 12 hours apart, for example 8 AM and 8 PM) in each infected ear unless the doctor has instructed otherwise. The best times to use the ear drops are in the morning and at night.
It is very important to use the ear drops for as long as the doctor has instructed, even if the symptoms improve. If Ofloxacin Otic Solution ear drops are not used for as long as the doctor has instructed, the infection may be more likely to return.
What if a dose is missed?
In patients with an Ear Canal Infection (“Swimmer’s Ear”), it is important that you take the drops every day. If you miss a dose which may have been scheduled for earlier in the day, (for example, 8 AM), you should take that day’s dose as soon as possible and then go back to your regular daily dosing schedule.
In patients with Middle Ear Infection, if a dose of Ofloxacin Otic Solution is missed, it should be given as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to the regular dosing schedule.
Do not use a double dose unless the doctor has instructed you to do so. If the infection is not improved after one week, you should consult your doctor. If you have two or more episodes of drainage within six months, it is recommended that you see your doctor for further evaluation.
What activities should be avoided while using Ofloxacin Otic Solution?
It is important that the infected ear(s) remain clean and dry. When bathing, avoid getting the infected ear(s) wet. Avoid swimming unless the doctor has instructed otherwise.
What are the possible side effects of Ofloxacin Otic Solution?
During the testing of Ofloxacin Otic Solution in external ear infections, the most common side effect was discomfort upon application which happened in 7% of patients. If the pain is severe, the medication should be stopped and you should contact your doctor. Other side effects were: itching (1%), earache (0.8%), and dizziness (0.4%).
During the testing of Ofloxacin Otic Solution in middle ear infections, the most common side effect was a bitter taste which happened in 7% of patients with a middle ear infection. This may occur when some of the medication passes from the middle ear to the back of the mouth. This side effect is not serious and there is no need to stop the medicine if this should happen. Other side effects which were found in 1% of the patients were: earache, itching, abnormal sensation, rash and dizziness.
Call your doctor for medical advice about side effects. You may report side effects to Akorn Operating Company LLC at 1-800-932-5676 or FDA at 1-800-FDA-1088.
If a rash or an allergic reaction to Ofloxacin Otic Solution occurs, stop using the product and contact your doctor.
DO NOT TAKE OFLOXACIN OTIC SOLUTION BY MOUTH.
If Ofloxacin Otic Solution is accidently swallowed or overdose occurs, call the doctor immediately. This medicine is available only with a doctor’s prescription. Use only as directed. Do not use this medicine if outdated.
If you wish to learn more about Ofloxacin Otic Solution ask the doctor or pharmacist.
HOW SUPPLIED
Plastic dropper bottle containing 5 mL and 10 mL
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Rx Only
Ofloxacin Otic Solution 0.3% is distributed by:
Akorn Operating Company LLC
Gurnee, IL 60031
This Patient Information has been approved by the U.S. Food and Drug Administration.
Close