Label: TRAZODONE HYDROCHLORIDE tablet
- NDC Code(s): 50111-450-01, 50111-450-02, 50111-560-01, 50111-560-02, view more
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 30, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRAZODONE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for TRAZODONE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. Trazodone hydrochloride tablets are not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
Close -
1 INDICATIONS AND USAGE
Trazodone hydrochloride tablets are indicated for the treatment of major depressive disorder (MDD) in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Dose Selection - An initial dose of 150 mg/day in divided doses is suggested. The dosage should be initiated at a low-dose and increased gradually, noting the clinical response and any ...
-
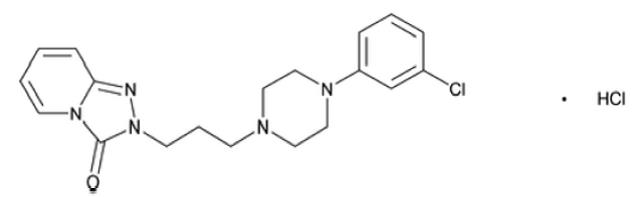
3 DOSAGE FORMS AND STRENGTHSTrazodone hydrochloride tablets, USP are available in the following strengths: 50 mg: White, round, compressed tablet, debossed “PLIVA 433” on one side and scored on the other side. 100 mg ...
-
4 CONTRAINDICATIONSTrazodone hydrochloride tablets are contraindicated in: Patients taking, or within 14 days of stopping, monoamine oxidase inhibitors (MAOIs), including MAOIs such as linezolid or intravenous ...
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Thoughts - and Behaviors in Pediatric and Young Adult Patients - In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: Suicidal Thoughts and Behavior in Children, Adolescents and Young Adults [see Boxed Warning and Warnings and ...
-
7 DRUG INTERACTIONS7.1 Drugs Having - Clinically Important Interactions with Trazodone Hydrochloride Tablets - Table 3: Clinically Important Drug Interactions with Trazodone Hydrochloride ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - Trazodone hydrochloride tablets are not a controlled substance. 9.2 Abuse - Although trazodone hydrochloride has not been systematically studied in preclinical or ...
-
10 OVERDOSAGEDeath from overdose has occurred in patients ingesting trazodone hydrochloride and other CNS depressant drugs concurrently (alcohol; alcohol and chloral hydrate and diazepam; amobarbital ...
-
11 DESCRIPTIONTrazodone hydrochloride tablets, USP for oral administration contain trazodone hydrochloride, USP a selective serotonin reuptake inhibitor and 5HT2 receptor antagonist. Trazodone hydrochloride ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of trazodone’s antidepressant action is not fully understood, but is thought to be related to its enhancement of serotonergic activity in the CNS ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No drug- or dose-related occurrence of carcinogenesis was evident in rats receiving trazodone in daily oral doses up ...
-
14 CLINICAL STUDIESThe efficacy and safety of trazodone hydrochloride were established from inpatient and outpatient trials of the trazodone immediate release formulation in the treatment of major depressive ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTrazodone hydrochloride tablets, USP are available as follows: 50 mg: White, round, compressed tablet, debossed “PLIVA 433” on one side and scored on the other side. Available in bottles of 100 ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide). Suicidal Thoughts and Behaviors - Advise patients and caregivers to look for the emergence of suicidality ...
-
MEDICATION GUIDE
Dispense with Medication Guide available at: www.tevausa.com/medguides - Trazodone Hydrochloride (traz' oh done hye'' droe klor' ide) Tablets - for oral use - What is the most important ...
-
Package/Label Display PanelNDC 50111-560-01 - TraZODONE Hydrochloride Tablets, USP - 50 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 Tablets
-
Package/Label Display PanelNDC 50111-561-01 - TraZODONE Hydrochloride Tablets, USP - 100 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 Tablets
-
Package/Label Display PanelNDC 50111-450-01 - TraZODONE Hydrochloride Tablets, USP - 150 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information