Label: CHOLESTYRAMINE powder, for suspension
- NDC Code(s): 49884-465-51, 49884-465-64, 49884-465-65, 49884-465-66, view more
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
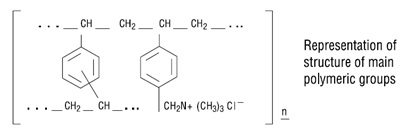
Cholestyramine for Oral Suspension USP, the chloride salt of a basic anion exchange resin, a cholesterol lowering agent, is intended for oral administration. Cholestyramine resin is quite ...
-
ACTIONS/CLINICAL PHARMACOLOGY
Cholesterol is probably the sole precursor of bile acids. During normal digestion, bile acids are secreted into the intestines. A major portion of the bile acids is absorbed from the intestinal ...
-
INDICATIONS AND USAGE
1) Cholestyramine for Oral Suspension USP is indicated as adjunctive therapy to diet for the reduction of elevated serum cholesterol in patients with primary hypercholesterolemia (elevated low ...
-
CONTRAINDICATIONS
Cholestyramine for oral suspension is contraindicated in patients with complete biliary obstruction where bile is not secreted into the intestine and in those individuals who have shown ...
-
WARNINGS
PHENYLKETONURICS: CHOLESTYRAMINE for ORAL SUSPENSION USP, LIGHT CONTAINS 14.0 mg PHENYLALANINE PER 5 GRAM DOSE.
-
PRECAUTIONS
General - Chronic use of cholestyramine resin may be associated with increased bleeding tendency due to hypoprothrombinemia associated with Vitamin K deficiency. This will usually respond ...
-
ADVERSE REACTIONS
The most common adverse reaction is constipation. When used as a cholesterol-lowering agent predisposing factors for most complaints of constipation are high dose and increased age (more than 60 ...
-
OVERDOSAGE
Overdosage with Cholestyramine has been reported in a patient taking 150% of the maximum recommended daily dosage for a period of several weeks. No ill effects were reported. Should an overdosage ...
-
DOSAGE AND ADMINISTRATIONThe recommended starting adult dose for all cholestyramine for oral suspension powdered products (Cholestyramine for Oral Suspension USP and Cholestyramine for Oral Suspension USP, Light) is one ...
-
HOW SUPPLIED
Cholestyramine for Oral Suspension USP is a yellow colored orange flavored powder available in cans containing 378 grams and in cartons of sixty 9 gram packets. Four grams of anhydrous ...
-
REFERENCES1.The Lipid Research Clinics Coronary Primary Prevention Trials Results: (I) Reduction in Incidence of Coronary Heart Disease; (II) The Relationship of Reduction in Incidence of Coronary Heart ...
-
PRINCIPAL DISPLAY PANEL, 60 PACKETS PER CARTON
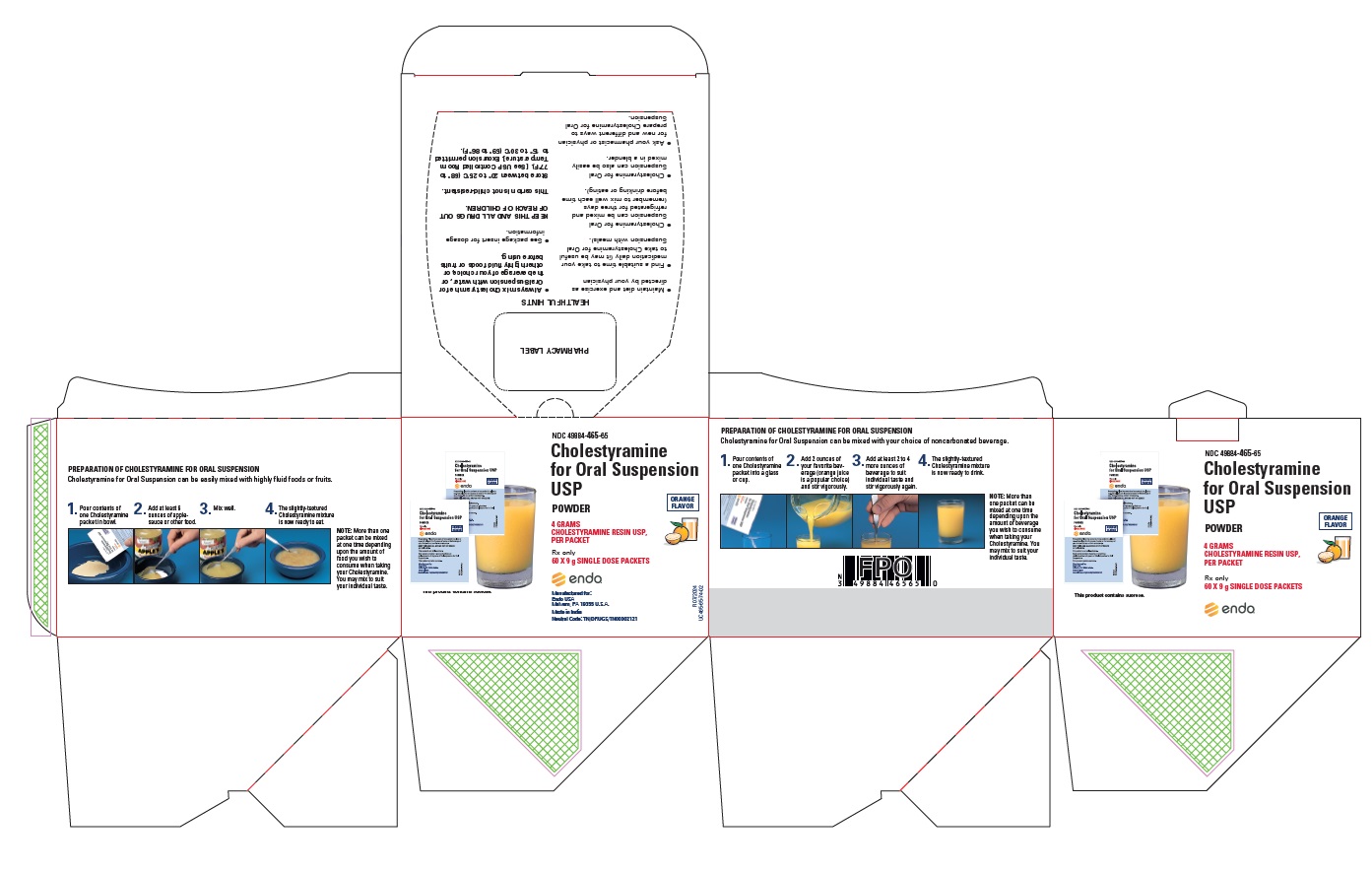
-
PRINCIPAL DISPLAY PANEL, 9 GRAM PACKET

-
PRINCIPAL DISPLAY PANEL, 378 g LABEL

-
PRINCIPAL DISPLAY PANEL, 5 GRAM PACKET

-
PRINCIPAL DISPLAY PANEL CARTON, 60 PACKETS PER CARTON
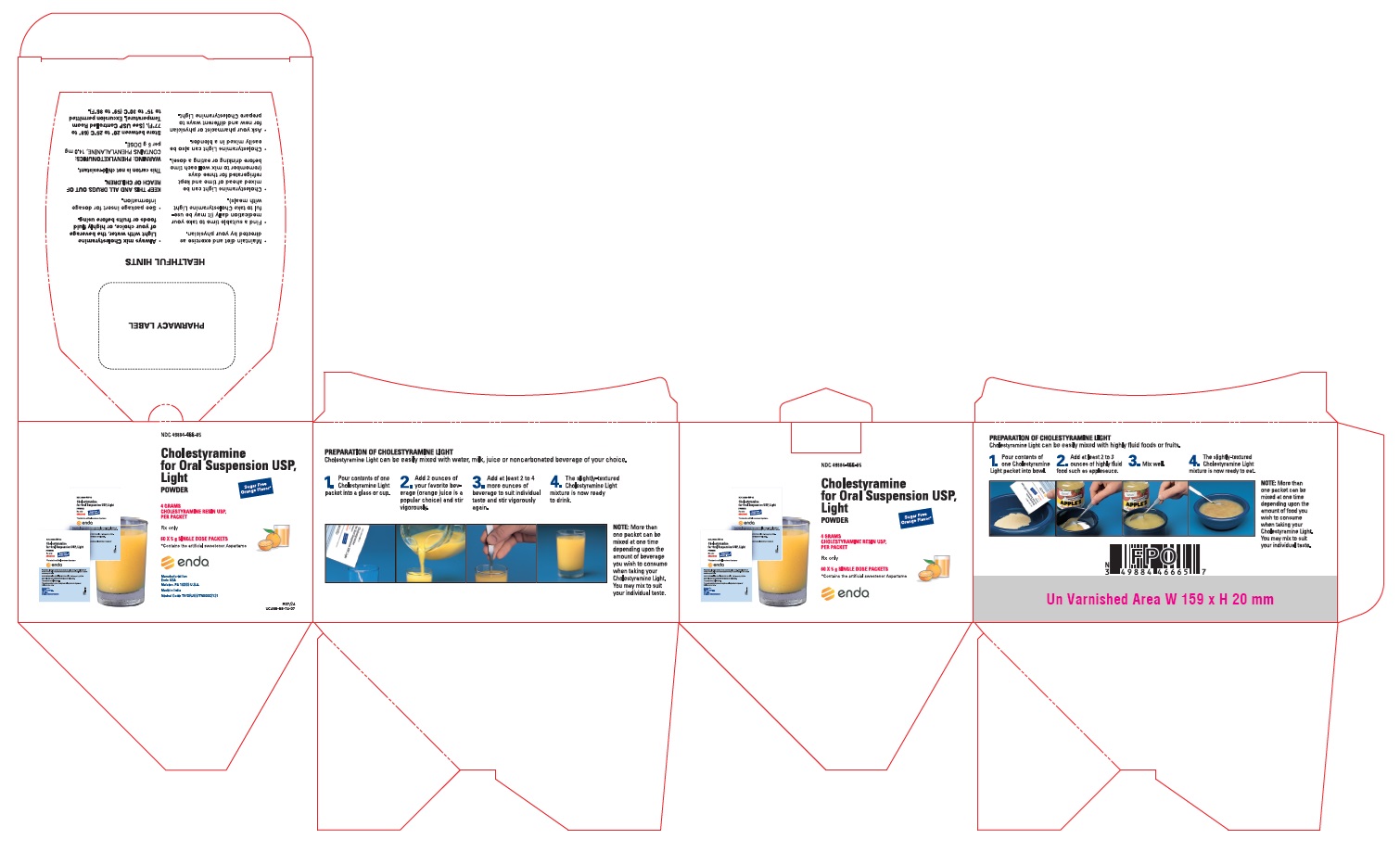
-
PRINCIPAL DISPLAY PANEL, 210 g LABEL - LIGHT

-
INGREDIENTS AND APPEARANCEProduct Information