Label: LABETALOL HCL- labetalol hydrochloride tablet, film coated
- NDC Code(s): 49884-122-01, 49884-122-05, 49884-123-01, 49884-123-05, view more
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
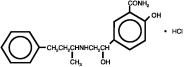
DESCRIPTIONLabetalol hydrochloride tablets, USP are adrenergic receptor blocking agents that have both selective alpha1-adrenergic and nonselective beta-adrenergic receptor blocking actions in a single ...
-
CLINICAL PHARMACOLOGYLabetalol HCl combines both selective, competitive, alpha1-adrenergic blocking and nonselective, competitive, beta-adrenergic blocking activity in a single substance. In man, the ratios of ...
-
INDICATIONS AND USAGELabetalol hydrochloride tablets, USP are indicated in the management of hypertension. Labetalol hydrochloride tablets, USP may be used alone or in combination with other antihypertensive agents ...
-
CONTRAINDICATIONSLabetalol hydrochloride tablets are contraindicated in bronchial asthma, overt cardiac failure, greater-than-first-degree heart block, cardiogenic shock, severe bradycardia, other conditions ...
-
WARNINGSHepatic Injury: Severe hepatocellular injury, confirmed by rechallenge in at least one case, occurs rarely with labetalol therapy.The hepatic injury is usually reversible, but hepatic necrosis ...
-
PRECAUTIONSGeneral - Impaired Hepatic Function: Labetalol hydrochloride tablets - should be used with caution in patients with impaired hepatic function since metabolism of the drug may be ...
-
ADVERSE REACTIONSMost adverse effects are mild and transient and occur early in the course of treatment. In controlled clinical trials of 3 to 4 months' duration, discontinuation of labetalol hydrochloride ...
-
OVERDOSAGEOverdosage with labetalol HCl causes excessive hypotension that is posture sensitive and, sometimes, excessive bradycardia. Patients should be placed supine and their legs raised if necessary to ...
-
DOSAGE AND ADMINISTRATIONDOSAGE MUST BE INDIVIDUALIZED. The recommended initial dosage is 100 mg twice daily whether used alone or added to a diuretic regimen. After 2 or 3 days, using standing blood pressure as an ...
-
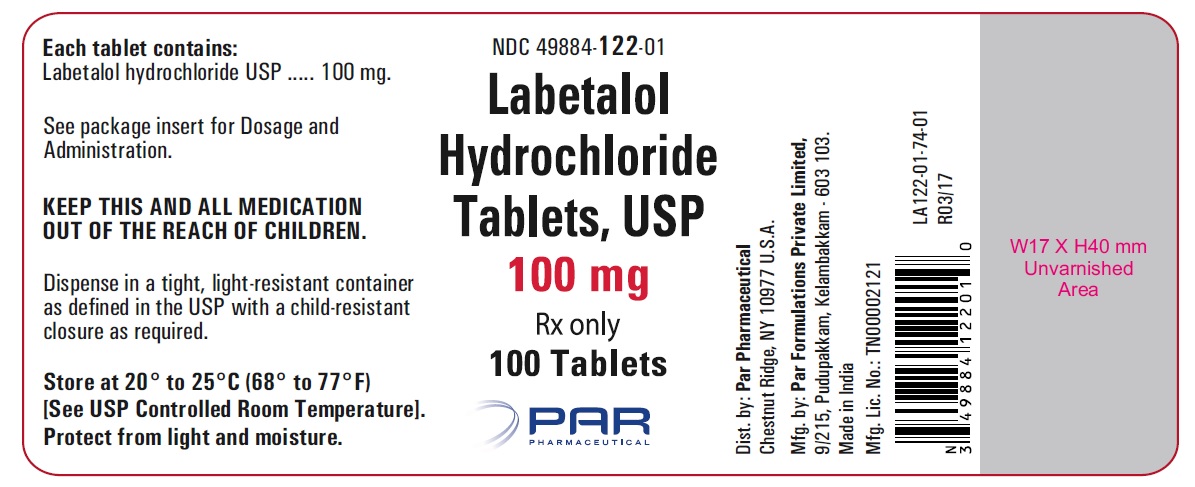
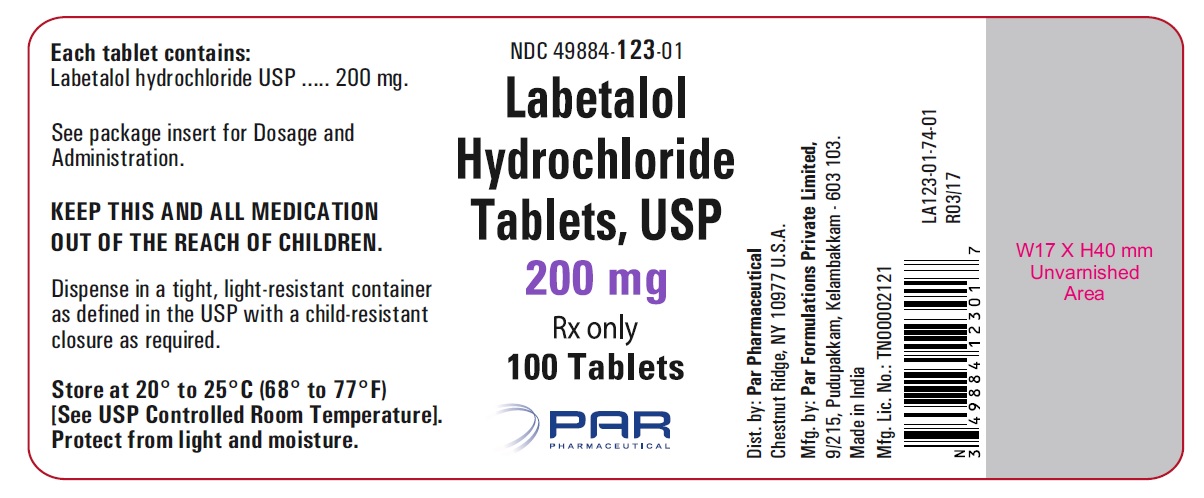
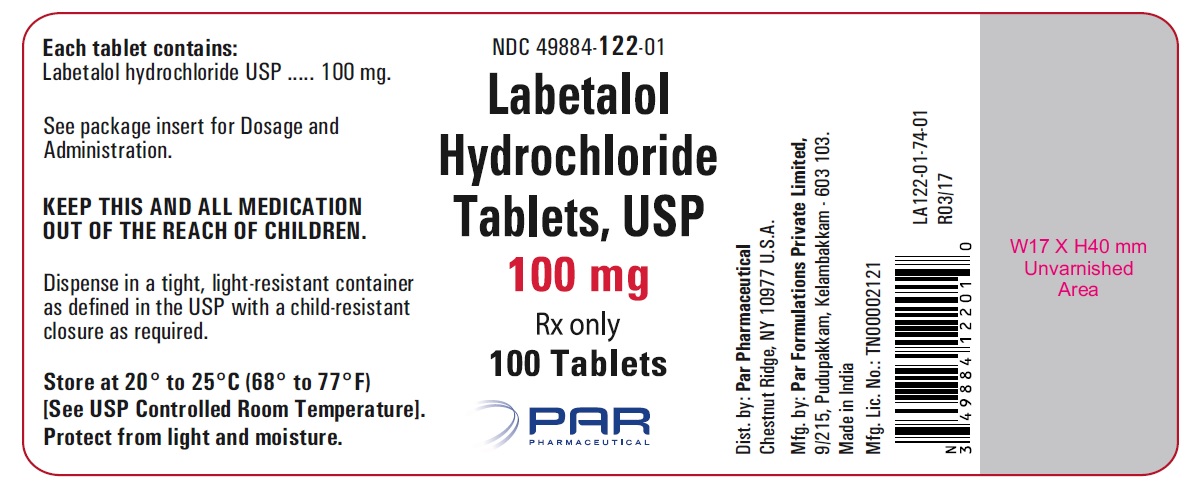
HOW SUPPLIEDLabetalol hydrochloride tablets, USP 100 mg, white, round, film-coated tablets with bisect, debossed “N” on top and “T” on bottom of the bisect on one side and "041" on the other side of the ...
-
PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL

-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information