Label: ISOSORBIDE DINITRATE tablet
- NDC Code(s): 49884-009-01, 49884-009-10, 49884-020-01, 49884-020-10, view more
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
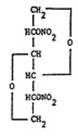
DESCRIPTIONIsosorbide dinitrate, an organic nitrate, is a vasodilator with effects on both arteries and veins. The chemical name for isosorbide dinitrate is 1,4:3,6-dianhydro-D-glucitol 2, 5-dinitrate. The ...
-
CLINICAL PHARMACOLOGYThe principal pharmacological action of isosorbide dinitrate is relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation ...
-
INDICATIONS AND USAGEIsosorbide dinitrate tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of immediate-release oral isosorbide dinitrate is not ...
-
CONTRAINDICATIONSIsosorbide dinitrate is contraindicated in patients who are allergic to isosorbide dinitrate or any of its ingredients. Do not use isosorbide dinitrate in patients who are taking certain drugs for ...
-
WARNINGSAmplification of the vasodilatory effects of isosorbide dinitrate by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied ...
-
PRECAUTIONSGeneral - Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide dinitrate. This drug should therefore be used with caution in patients who may be ...
-
ADVERSE REACTIONSAdverse reactions to isosorbide dinitrate are generally dose-related, and almost all of these reactions are the result of isosorbide dinitrate's activity as a vasodilator. Headache, which may be ...
-
OVERDOSAGEHemodynamic Effects - The ill effects of isosorbide dinitrate overdose are generally the results of isosorbide dinitrate's capacity to induce vasodilatation, venous pooling, reduced cardiac ...
-
DOSAGE AND ADMINISTRATIONAs noted under "CLINICAL PHARMACOLOGY," multiple-dose studies with isosorbide dinitrate and other nitrates have shown that maintenance of continuous 24-hour plasma levels results in refractory ...
-
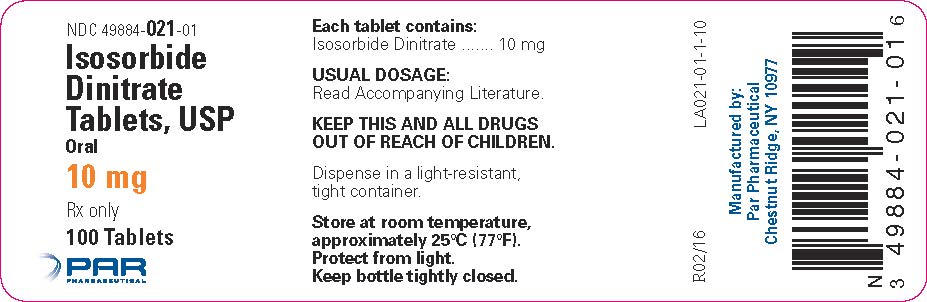
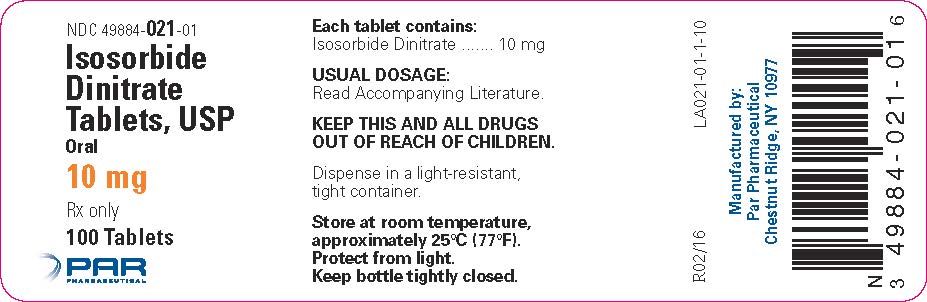
HOW SUPPLIEDIsosorbide Dinitrate Tablets, USP are available as follows: 5 mg oral, white, round, bisected tablets, debossed with "PAR 020" on one side and plain on the other side. Bottles of 100 NDC ...
-
PRINCIPAL DISPLAY PANEL – 5 MG/100 TABLET CONTAINER LABEL

-
PRINCIPAL DISPLAY PANEL – 10 MG/100 TABLET CONTAINER LABEL

-
PRINCIPAL DISPLAY PANEL – 20 MG/100 TABLET CONTAINER LABEL
-
PRINCIPAL DISPLAY PANEL – 30 MG/100 TABLET CONTAINER LABEL

-
INGREDIENTS AND APPEARANCEProduct Information