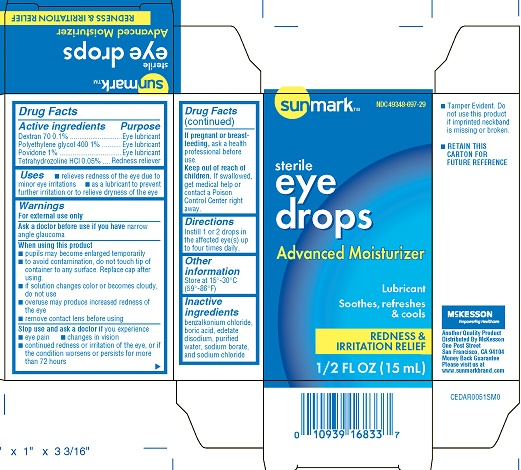
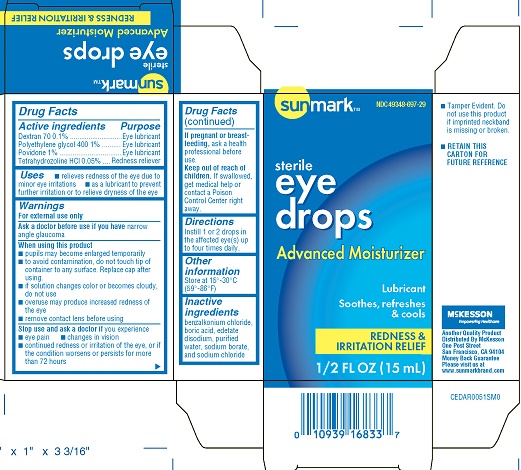
Label: SUNMARK EYE DROPS ADVANCED MOISTURIZER/LUBRICANT- dextran, polyethylene glycol 400, povidone, tetrahydrozoline solution/...view full title
- NDC Code(s): 49348-697-29
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientsDextran 70 0.1% povidone 1% Polyethylene glycol 400 1% Tetrahydrozoline HCL 0.05%
-
Purpose Eye Lubricant - Redness reliever
-
Usesrelieves redness of the eye due to minor eye irritations - as a lubricant to prevent further irritation or to relieve dryness of the eye
-
WarningsFor external use only - Ask a doctor before use if you have - narrow angle glucoma - When using this product - pupils may become enlarged temporarily - to avoid contamination, do not touch tip of ...
-
DirectionsInstill 1 or 2 drops in the affected eye(s) up to four times daily.
-
Other informationStore at 15 - o- 30 - oC (59 - o- 86 - oF)
-
Inactive Ingredientsbenzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate, and sodium chloride
-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information