Label: DILTIAZEM HYDROCHLORIDE capsule, extended release
- NDC Code(s): 47335-675-13, 47335-675-18, 47335-675-19, 47335-675-81, view more
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
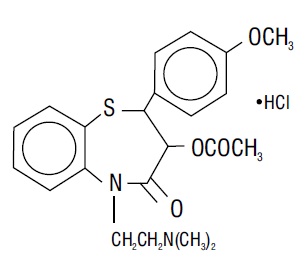
DESCRIPTIONDiltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one ...
-
CLINICAL PHARMACOLOGYThe therapeutic effects of diltiazem hydrochloride are believed to be related to its ability to inhibit the cellular influx of calcium ions during membrane depolarization of cardiac and vascular ...
-
INDICATIONS AND USAGEDiltiazem hydrochloride extended-release capsules (Once-a-day dosage) are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive ...
-
CONTRAINDICATIONSDiltiazem is contraindicated in (1) patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker, (2) patients with second- or third-degree AV block except in ...
-
WARNINGSCardiac Conduction: Diltiazem prolongs AV node refractory periods without significantly prolonging sinus node recovery time, except in patients with sick sinus syndrome. This effect may rarely ...
-
PRECAUTIONSGeneral - Diltiazem hydrochloride is extensively metabolized by the liver and excreted by the kidneys and in bile. Laboratory parameters of renal and hepatic function should be monitored at ...
-
ADVERSE REACTIONSSerious adverse reactions have been rare in studies carried out to date, but it should be recognized that patients with impaired ventricular function and cardiac conduction abnormalities have ...
-
OVERDOSAGEThe oral LD50s in mice and rats range from 415 to 740 mg/kg and from 560 to 810 mg/kg, respectively. The intravenous LD50s in these species were 60 and 38 mg/kg, respectively. The oral LD50 in ...
-
DOSAGE AND ADMINISTRATIONPatients controlled on diltiazem alone or in combination with other medications may be switched to diltiazem hydrochloride extended-release capsules at the nearest equivalent total daily dose ...
-
HOW SUPPLIEDDiltiazem Hydrochloride Extended-Release Capsules, USP (Once-a-Day Dosage) Strength Quantity NDC Number Description - 120 mg - Bottle of 30 CRC - Bottle of 90 CRC - Bottle of 90 ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Sun Pharmaceutical Industries, Inc. Cranbury, NJ 08512 - Manufactured by: Sun Pharmaceutical Industries Ltd. Halol-Baroda Highway, Halol-389 350, Gujarat, India. ISS. 01/2023 ...
-
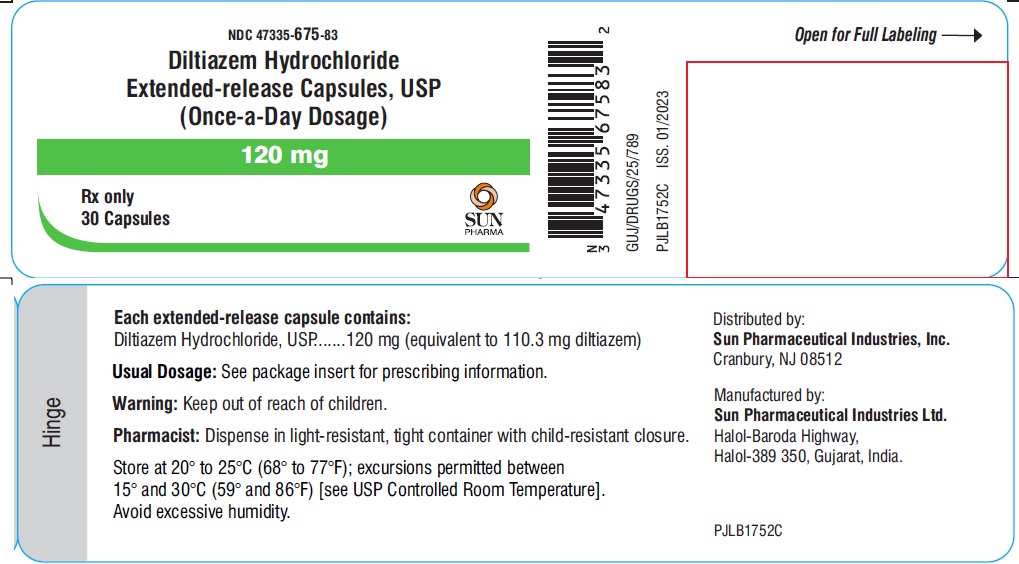
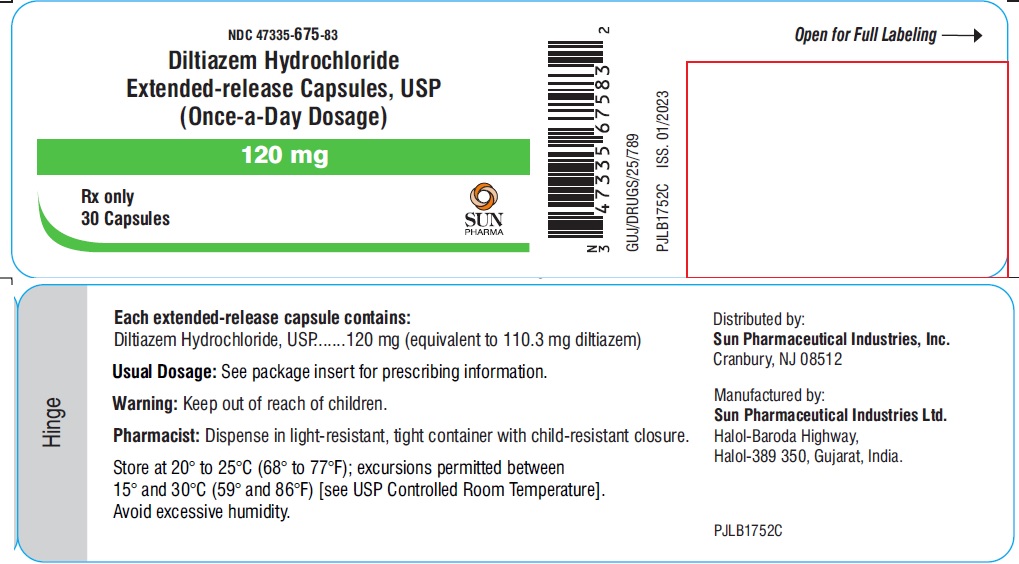
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 120 MGNDC 47335-675-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 120 mg - Rx only - 30 Capsules - SUN PHARMA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 180 MGNDC 47335-676-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 180 mg - Rx only - 30 Capsules - SUN PHARMA
-
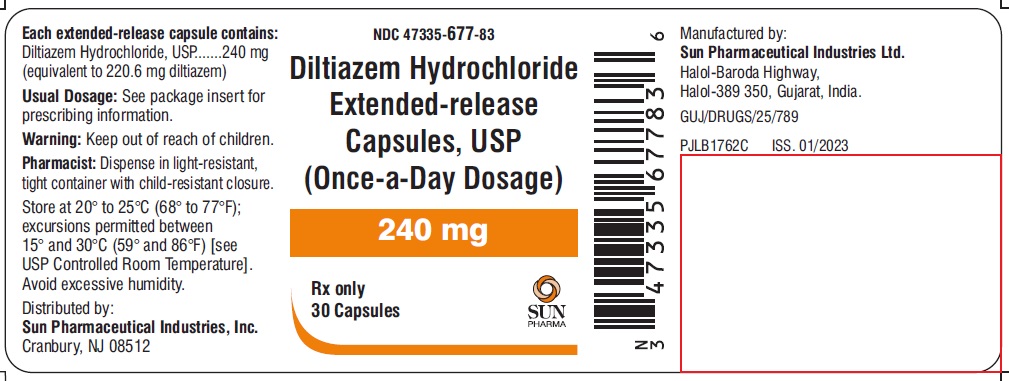
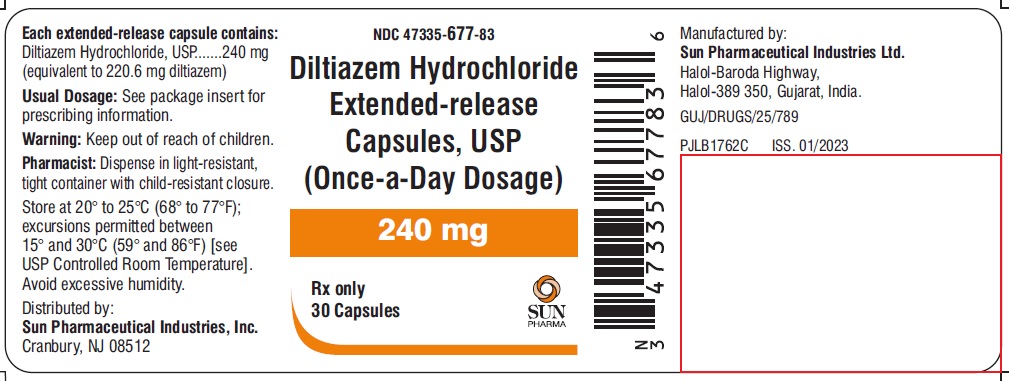
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 240 MGNDC 47335-677-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 240 mg - Rx only - 30 Capsules - SUN PHARMA
-
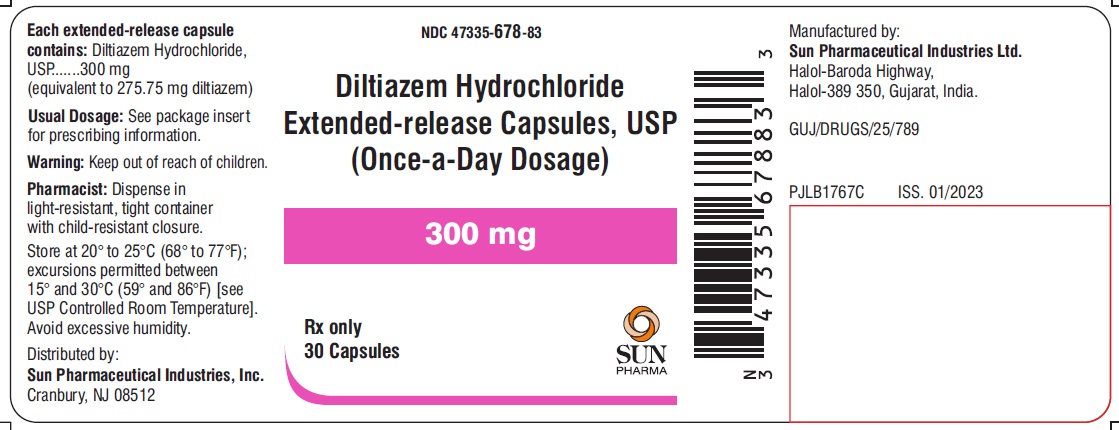
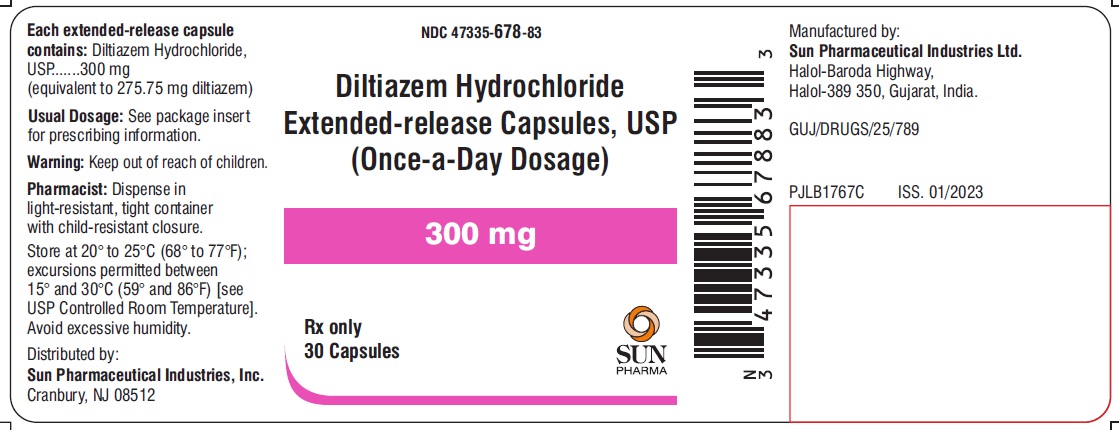
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 300 MGNDC 47335-678-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 300 mg - Rx only - 30 Capsules - SUN PHARMA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 360 MGNDC 47335-679-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 360 mg - Rx only - 30 Capsules - SUN PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information