Label: DILTIAZEM HYDROCHLORIDE capsule, extended release
- NDC Code(s): 47335-669-13, 47335-669-18, 47335-669-19, 47335-669-81, view more
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
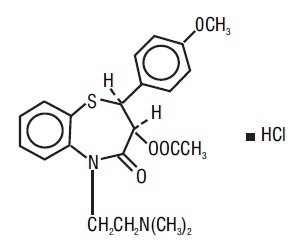
DESCRIPTIONDiltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker). Chemically, diltiazem hydrochloride is ...
-
CLINICAL PHARMACOLOGYThe therapeutic effects of diltiazem hydrochloride are believed to be related to its ability to inhibit the cellular influx of calcium ions during membrane depolarization of cardiac and vascular ...
-
INDICATIONS AND USAGEHypertension: Diltiazem hydrochloride extended-release capsules are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive ...
-
CONTRAINDICATIONSDiltiazem is contraindicated in: • Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker - • Patients with second- or third-degree AV block except in the ...
-
WARNINGS1. Cardiac Conduction: Diltiazem hydrochloride prolongs AV node refractory periods without significantly prolonging sinus node recovery time, except in patients with sick sinus syndrome. This ...
-
PRECAUTIONSGeneral - Diltiazem hydrochloride is extensively metabolized by the liver and excreted by the kidneys and in bile. As with any drug given over prolonged periods, laboratory parameters of renal ...
-
ADVERSE REACTIONSSerious adverse reactions have been rare in studies with diltiazem, as well as with other diltiazem formulations. It should be recognized that patients with impaired ventricular function and ...
-
OVERDOSAGEThe oral LD50s in mice and rats range from 415 to 740 mg/kg and from 560 to 810 mg/kg, respectively. The intravenous LD50s in these species were 60 and 38 mg/kg, respectively. The oral LD50 in ...
-
DOSAGE AND ADMINISTRATIONHypertension: Dosage needs to be adjusted by titration to individual patient needs. When used as monotherapy, usual starting doses are 120 to 240 mg once daily. Maximum antihypertensive effect ...
-
HOW SUPPLIEDDiltiazem Hydrochloride Extended-Release Capsules, USP - StrengthDescriptionQuantityNDC# 120 mg#2 purple colored cap and body with “669” imprinted in black ink on cap and body ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Sun Pharmaceutical Industries, Inc. Cranbury, NJ 08512 - Manufactured by: Sun Pharmaceutical Industries Ltd. Halol-Baroda Highway, Halol-389 350, Gujarat, India. ISS ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 120 MGNDC 47335-669-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 120 mg - Rx only - 30 Capsules - SUN PHARMA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 180 MGNDC 47335-670-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 180 mg - Rx only - 30 Capsules - SUN PHARMA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 240 MGNDC 47335-671-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 240 mg - Rx only - 30 Capsules - SUN PHARMA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 300 MGNDC 47335-672-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 300 mg - Rx only - 30 Capsules - SUN PHARMA
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 360 MGNDC 47335-673-83 - Diltiazem Hydrochloride Extended-release Capsules, USP - (Once-a-Day Dosage) 360 mg - Rx only - 30 Capsules - SUN PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information