Label: CETIRIZINE HYDROCHLORIDE tablet, chewable
- NDC Code(s): 47335-343-83, 47335-343-88, 47335-344-83, 47335-344-88
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
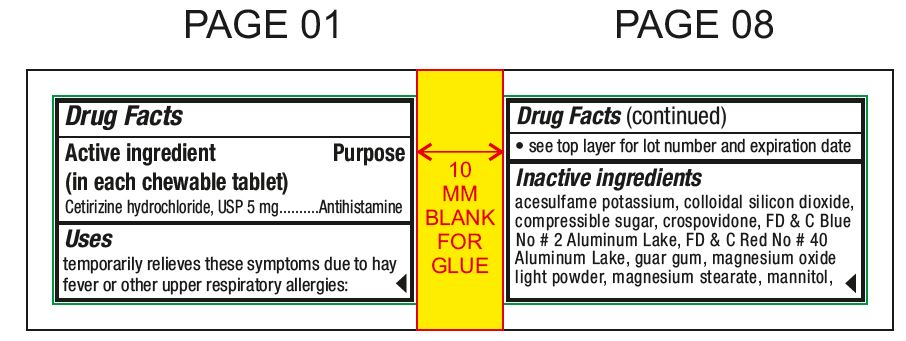
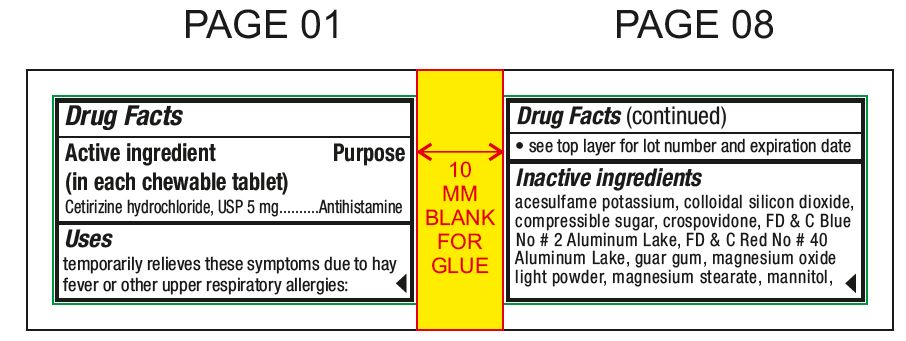
Active ingredient (in each chewable tablet)For 5 mg:Cetirizine hydrochloride, USP 5 mg - For 10 mg:Cetirizine hydrochloride, USP 10 mg
-
PurposeAntihistamine
-
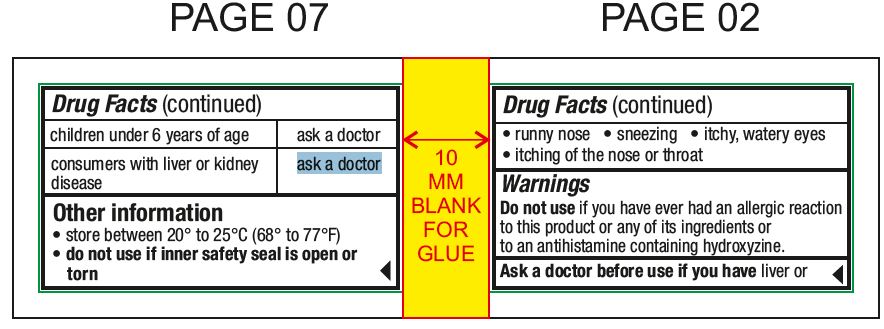
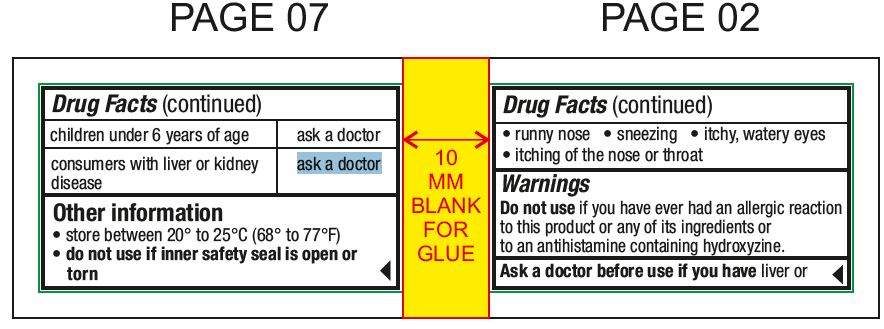
Usestemporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose - sneezing - itchy, watery eyes - itching of the nose or throat
- SPL UNCLASSIFIED SECTION
- Warnings
-
Do not useif you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
-
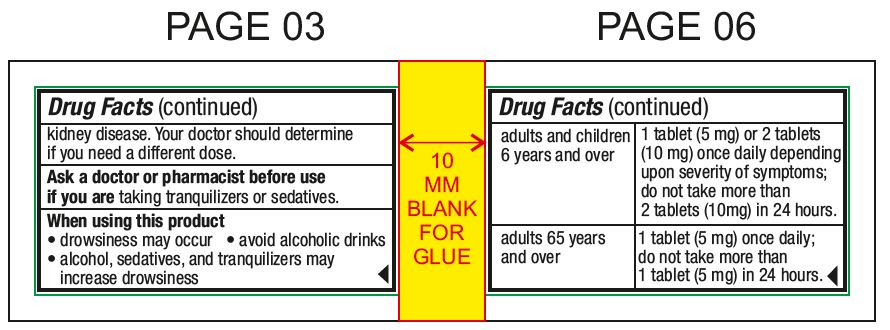
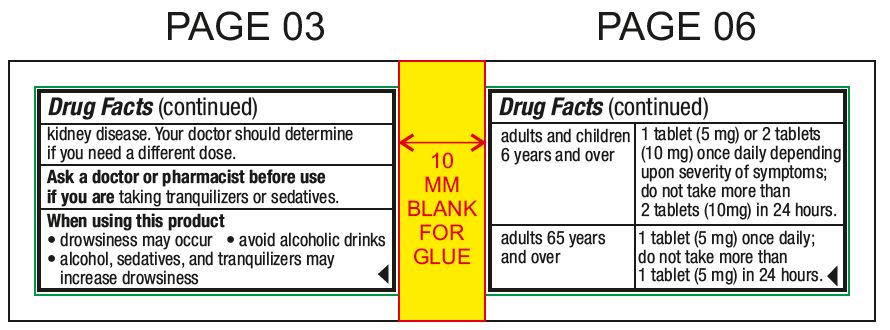
Ask a doctor before use if you haveliver or kidney disease. Your doctor should determine if you need a different dose.
-
Ask a doctor or pharmacist before use ifyou are taking tranquilizers or sedatives.
-
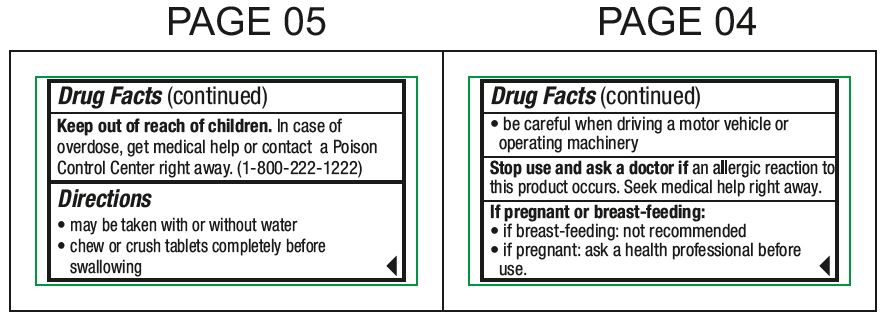
When using this productdrowsiness may occur - avoid alcoholic drinks - alcohol, sedatives, and tranquilizers may increase drowsiness - be careful when driving a motor vehicle or operating machinery
-
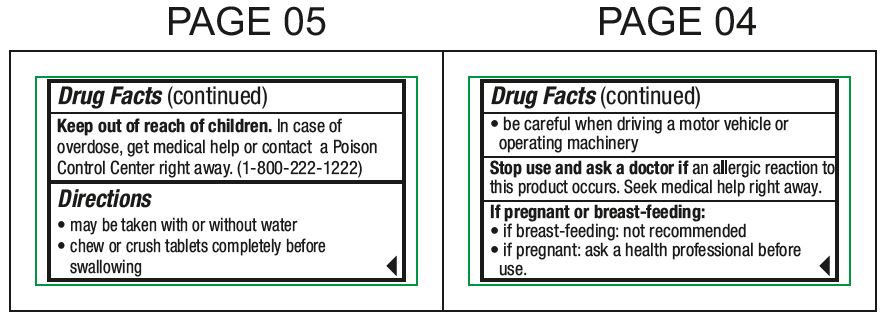
Stop use and ask doctor ifan allergic reaction to this product occurs. Seek medical help right away.
-
If pregnant or breast-feeding:if breast-feeding: not recommended - if pregnant: ask a health professional before use.
-
Keep out of reach of childrenIn case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
-
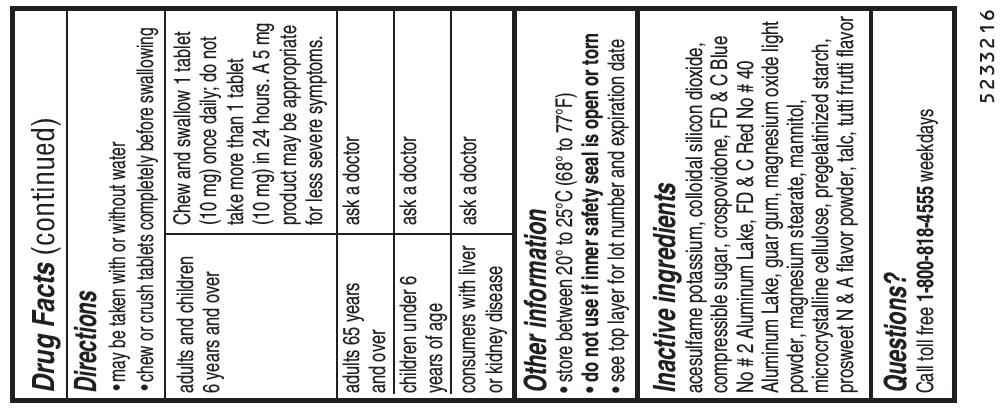
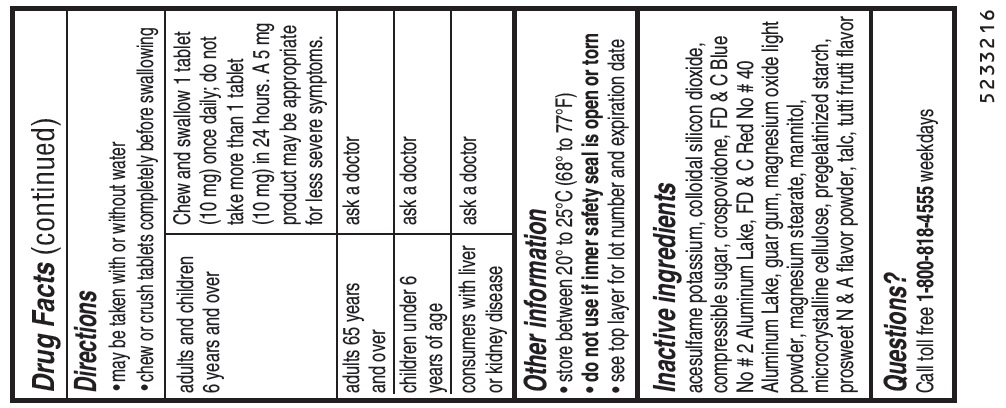
Directionsmay be taken with or without water - chew or crush tablets completely before swallowing - For 5 mg: adults and children 6 years and over1 tablet (5 mg) or 2 tablets (10 mg) once daily ...
-
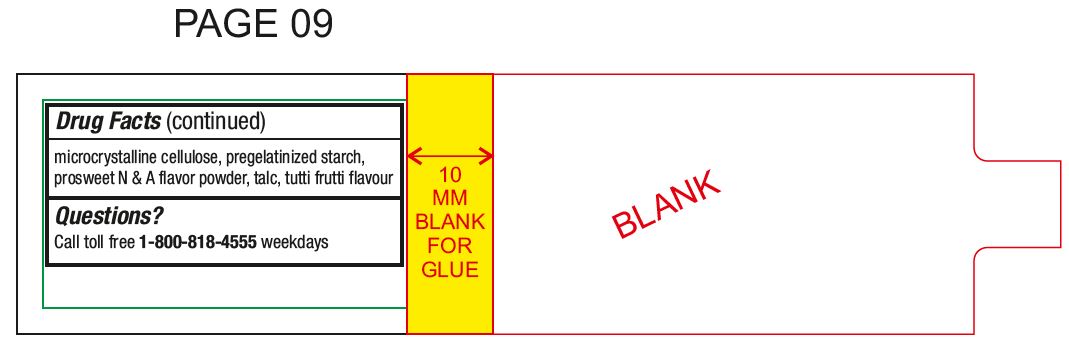
Other informationstore between 20° to 25°C (68° to 77°F) do not use if inner safety seal is open or torn - see top layer for lot number and expiration date
-
Inactive ingredientsacesulfame potassium, colloidal silicon dioxide, compressible sugar, crospovidone, FD & C Blue No # 2 Aluminum Lake, FD & C Red No # 40 Aluminum Lake, guar gum, magnesium oxide light powder ...
-
Questions?Call toll free 1-800-818-4555 weekdays
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELFor 5 mg Allergy: Original Prescription Strength - NDC 47335-343-83 - Cetirizine Hydrochloride Chewable Tablets - 5 mg - ALLERGY - Antihistamine - Indoor + Outdoor Allergies - Actual ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELFor 10 mg Allergy: Original Prescription Strength - NDC 47335-344-83 - Cetirizine Hydrochloride Chewable Tablets - 10 mg - ALLERGY - Antihistamine - Indoor + Outdoor Allergies - Actual ...
-
INGREDIENTS AND APPEARANCEProduct Information