Label: CARBIDOPA, LEVODOPA AND ENTACAPONE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 47335-001-88, 47335-002-88, 47335-003-88, 47335-004-88, view more - Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated March 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CARBIDOPA, LEVODOPA AND ENTACAPONE TABLETS safely and effectively. See full prescribing information for CARBIDOPA, LEVODOPA AND ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECarbidopa, levodopa and entacapone tablets, a combination drug consisting of levodopa, carbidopa (dopa decarboxylase inhibitor), and entacapone (catechol-O-methyltransferase-COMT inhibitor) is ...
-
2 DOSAGE AND ADMINISTRATIONCarbidopa, levodopa and entacapone tablets should be used as a substitute for patients already stabilized on equivalent doses of carbidopa/levodopa and entacapone. However, some patients who have ...
-
3 DOSAGE FORMS AND STRENGTHSEach carbidopa, levodopa and entacapone tablet, provided in 6 single-dose strengths, contains carbidopa and levodopa in a 1:4 ratio and a 200 mg dose of entacapone. Carbidopa, levodopa and ...
-
4 CONTRAINDICATIONSCarbidopa, levodopa and entacapone tablets are contraindicated in patients: Taking nonselective monoamine oxidase (MAO) inhibitors (e.g., phenelzine and tranylcypromine). These nonselective MAO ...
-
5 WARNINGS AND PRECAUTIONSThe following adverse reactions described in this section are related to at least one of the components of carbidopa, levodopa and entacapone tablets (i.e., levodopa, carbidopa, and/or entacapone ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in the Warnings and Precautions sections of labeling: Falling Asleep During Activities of Daily Living and Somnolence [seeWarnings ...
-
7 DRUG INTERACTIONS7.1 MAO Inhibitors - Patients receiving nonselective MAO inhibitors and carbidopa, levodopa and entacapone may be at risk of increased adrenergic tone. Therefore, the use of carbidopa, levodopa ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - There are no adequate and well-controlled studies in pregnant women. It has been reported from individual cases that levodopa crosses the human placental ...
-
10 OVERDOSAGE10.1 Signs and Symptoms of Overdosage - There are very few cases of overdose with levodopa reported in the published literature. Based on the available information, the acute symptoms of levodopa ...
-
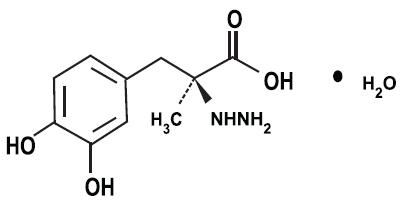
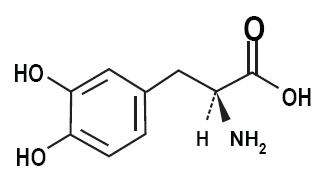
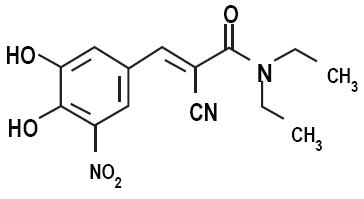
11 DESCRIPTIONCarbidopa, levodopa and entacapone tablets are combination of carbidopa, levodopa, and entacapone for the treatment of Parkinson’s disease. Carbidopa, an inhibitor of aromatic amino acid ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Levodopa - Current evidence indicates that symptoms of Parkinson’s disease are related to depletion of dopamine in the corpus striatum. Administration of dopamine is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility - Carcinogenesis - In rats, oral administration of carbidopa-levodopa for 2 years resulted in no evidence of carcinogenicity at doses of ...
-
14 CLINICAL STUDIESThe effectiveness of entacapone as an adjunct to levodopa in the treatment of Parkinson’s disease was established in three 24-week multicenter, randomized, double‑blind, placebo‑controlled studies ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCarbidopa, levodopa and entacapone tablets are supplied as film-coated tablets for oral administration in the following six strengths: Carbidopa, levodopa and entacapone tablets 12.5 mg/50 ...
-
17 PATIENT COUNSELING INFORMATIONFalling Asleep During Activities of Daily Living and Somnolence - Advise patients about the potential for sedating effects associated with carbidopa, levodopa and entacapone tablets including ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 47335-001-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP........ 12.5 mg - Levodopa USP ......... 50 mg - And - Entacapone ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-showboxNDC 47335-001-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP........ 12.5 mg - Levodopa USP ......... 50 mg - And - Entacapone ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-SHOWBOXNDC 47335-002-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP ..............18.75 mg - Levodopa USP ................... 75 mg ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 47335-002-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP ..............18.75 mg - Levodopa USP ................... 75 mg - And ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-SHOWBOXNDC 47335-003-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP................................. 25 mg - Levodopa ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 47335-003-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP................................. 25 mg - Levodopa ...
-
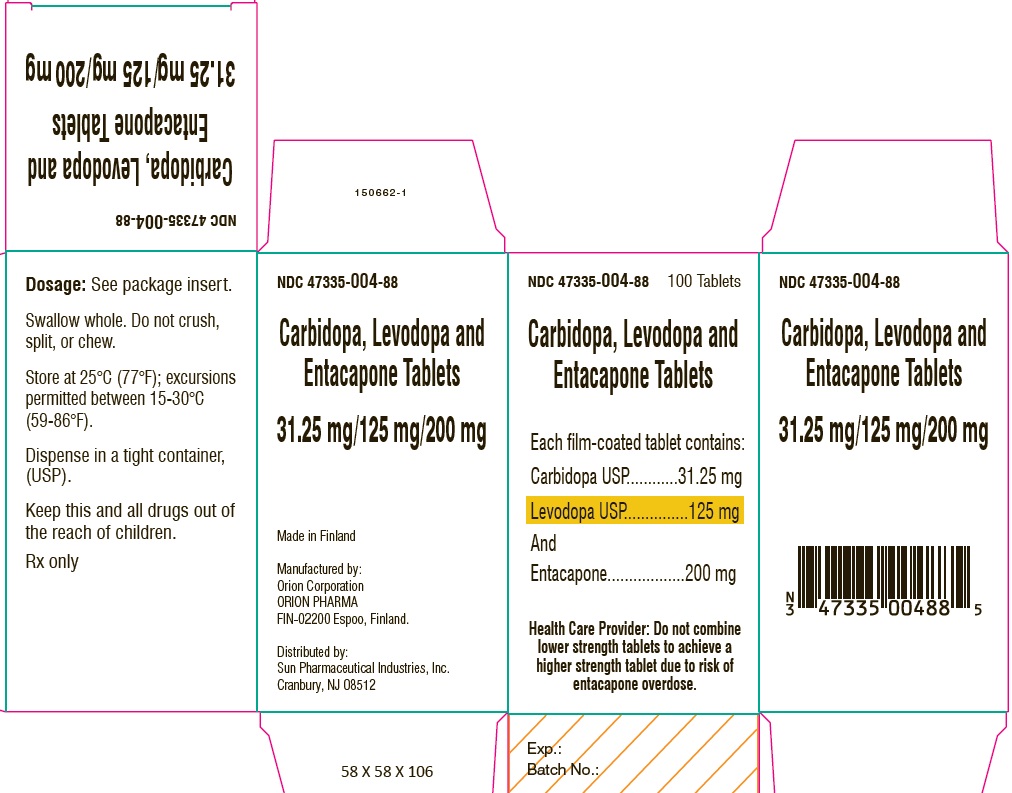
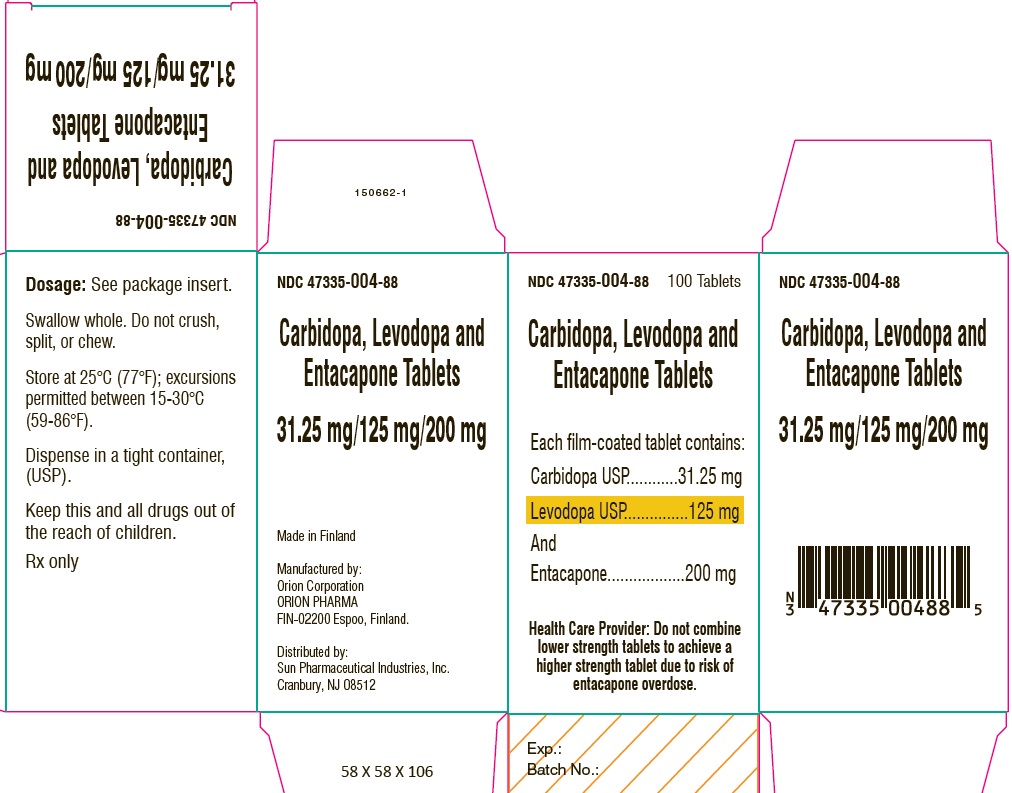
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-SHOWBOXNDC 47335-004-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP.................................. 31.25 mg - Levodopa ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 47335-004-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP.................................. 31.25 mg - Levodopa ...
-
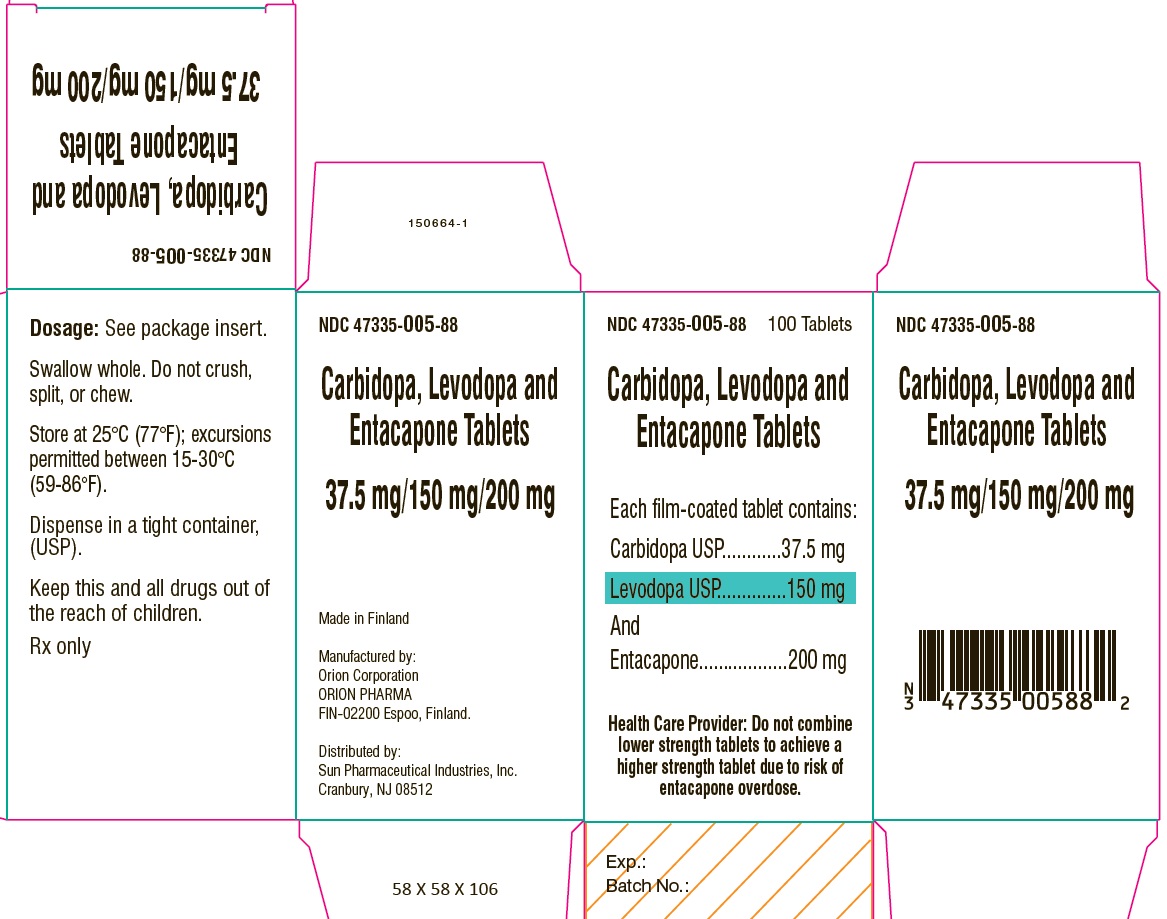
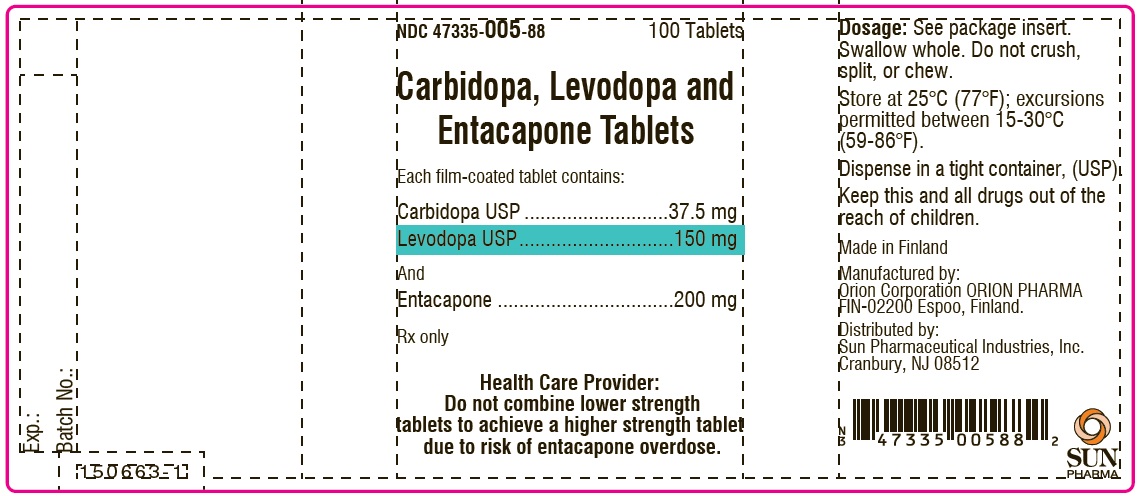
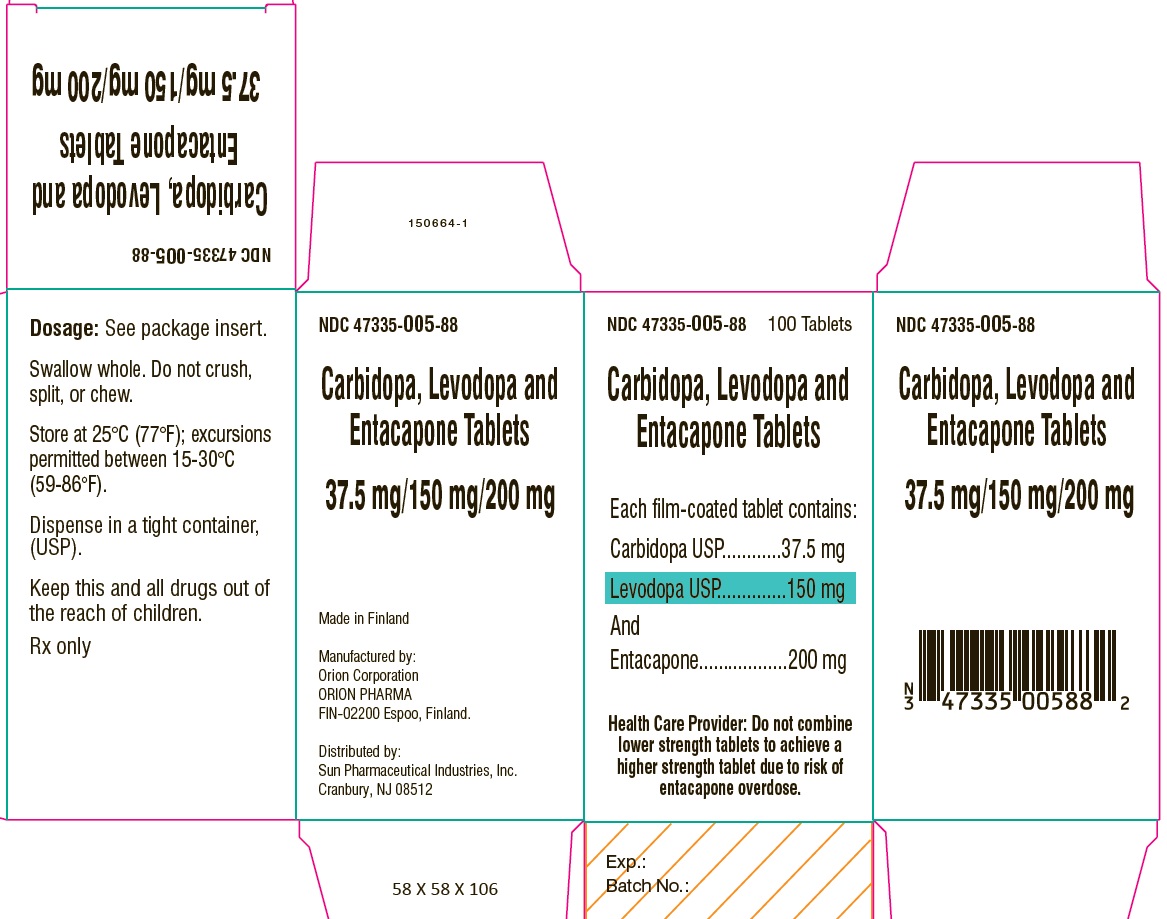
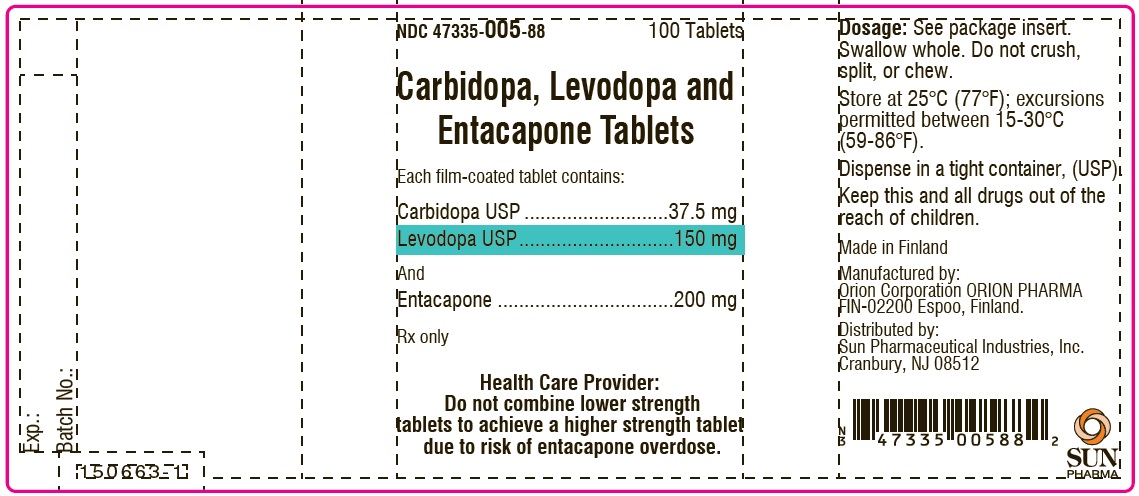
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-SHOWBOXNDC 47335-005-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP.................................. 37.5 mg - Levodopa ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 47335-005-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP.................................. 37.5 mg - Levodopa ...
-
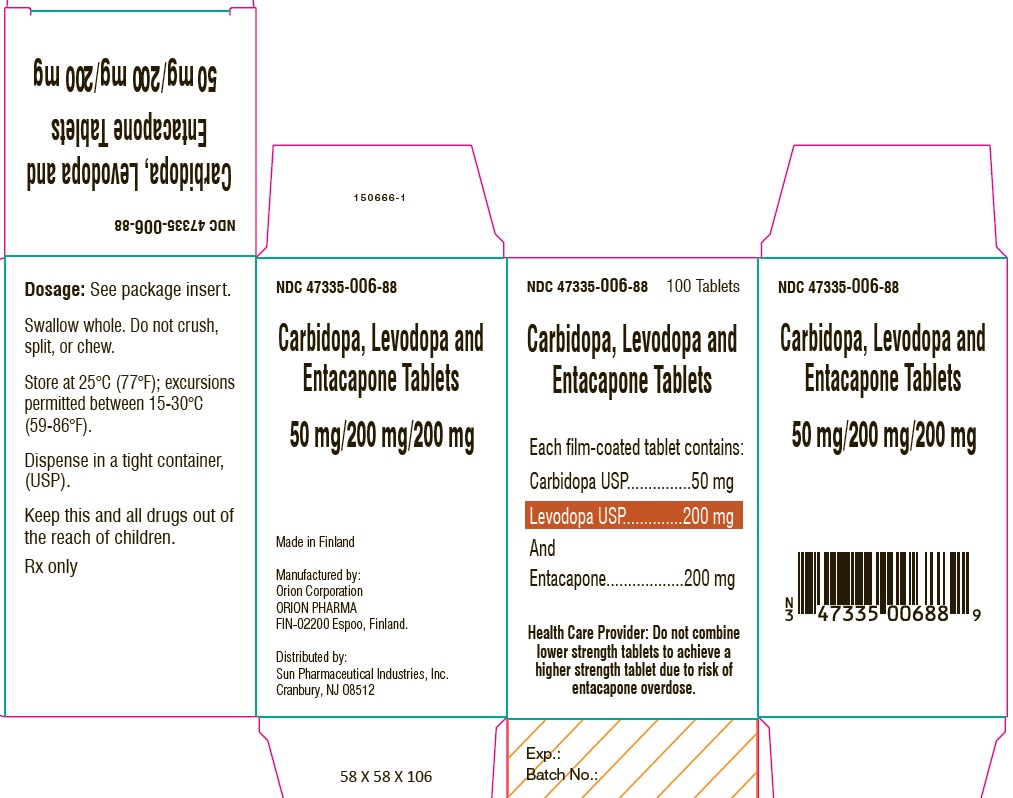
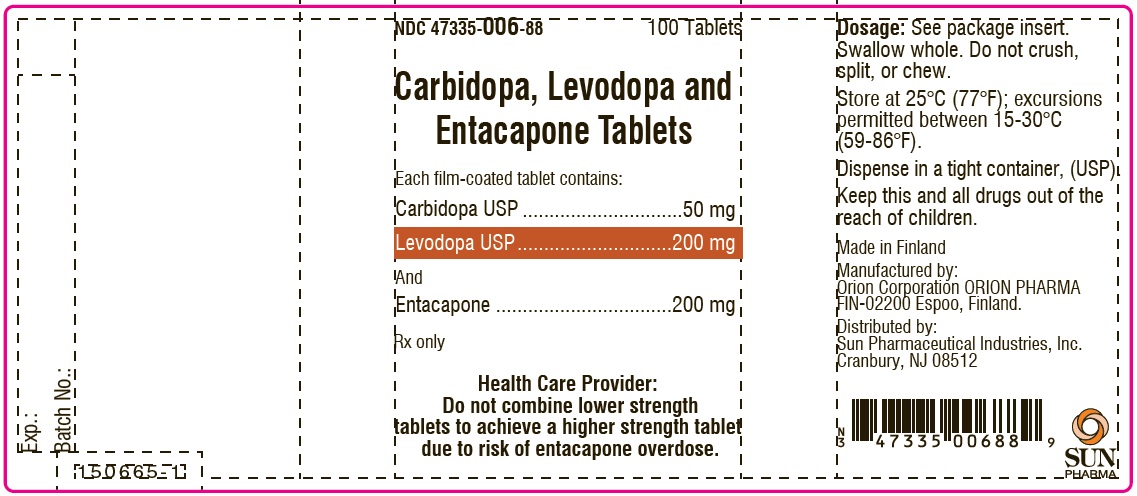
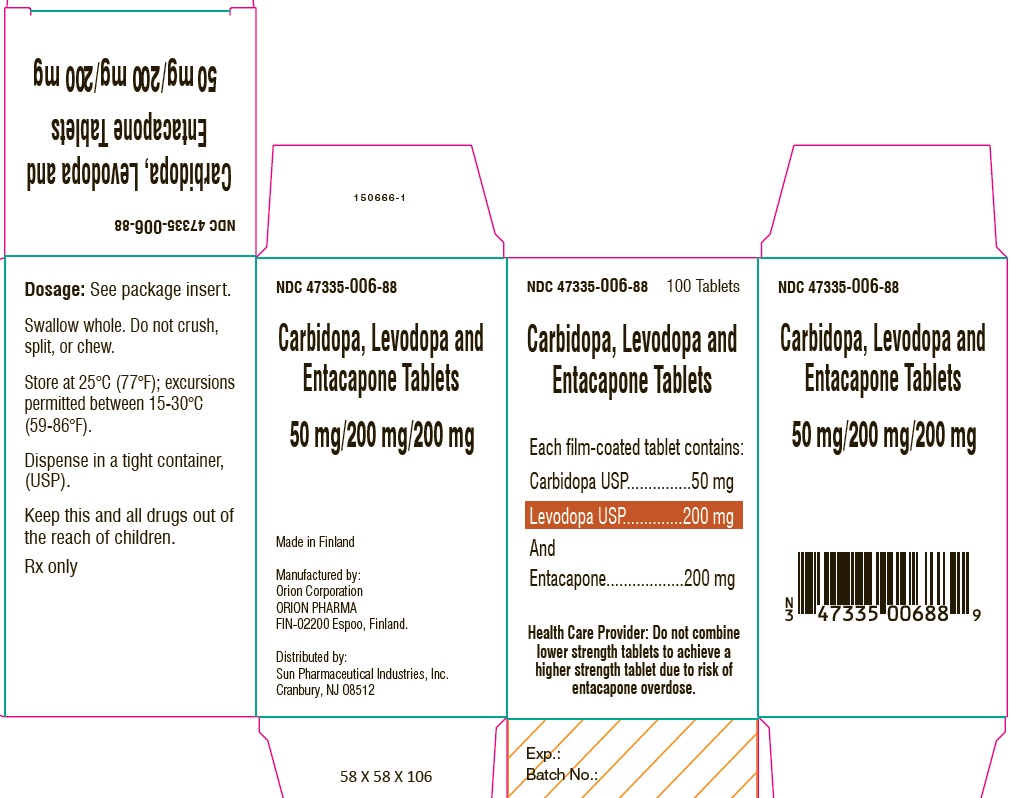
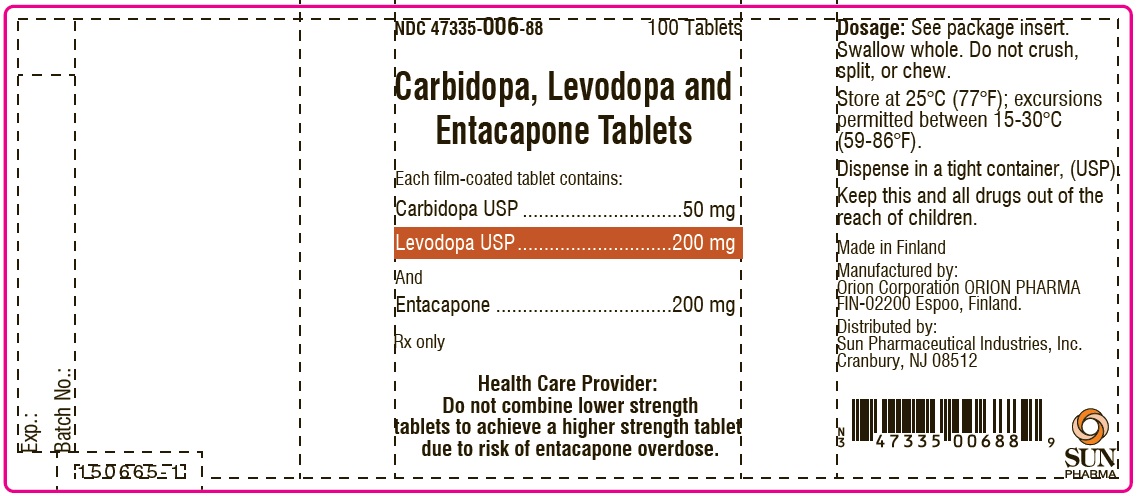
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-SHOWBOXNDC 47335-006-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP.................................. 50 mg - Levodopa ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 47335-006-88 - 100 Tablets - Carbidopa, Levodopa and Entacapone Tablets - Each film-coated tablet contains: Carbidopa USP.................................. 50 mg - Levodopa ...
-
INGREDIENTS AND APPEARANCEProduct Information